Research Areas
Molecular regulation of T cell responses at mucosal sites

Role of the transcriptional regulator Blimp1 in controlling effector and regulatory T cell at the intestinal mucosa.
Shaping of the intestinal commensal microbiota by T cells.
One current focus of the laboratory is the study of the mechanisms by which the transcription factor B-lymphocyte-induced maturation protein-1 (Blimp-1/PRD1-BF1)/PRDM1 regulates T helper cell differentiation and function. Blimp-1 is a highly evolutionary conserved protein and one of the founding members of the PRDM (PR domain containing genes with zinc fingers) family of proteins. PRDM proteins are characterized by the presence of a SET methyltransferase-related N-terminal PR domain and multiple zinc fingers that mediate sequence-specific DNA binding and protein-protein interactions. PRDM family members control gene expression through modification of the chromatin state at target gene promoters,using either enzymatic activity toward histones or recruitment of interacting partners. Blimp-1 stands out among other PRDM proteins because of its in role in regulating cell fate decisions in many different lineages, ranging from intestinal enterocytes to skin keratinocytes, and many different hematopoietic lineages.
The crucial role of Blimp-1 in hematopoietic cells was first discovered in B lymphocytes, in which Blimp-1 is required and sufficient to drive the functional differentiation that leads to antibody production. Blimp-1’s role in T lymphocytes was unknown until very recently, when we and others demonstrated that Blimp-1 is required for proper functioning of T lymphocytes, such that mice with T cell-specific deletion of Blimp-1 (Blimp-1CKO mice) spontaneously develop a severe form of inflammatory bowel disease (IBD), which is associated with accumulation of CD4+ T cells specialized in producing the inflammatory cytokine IL-17.
Using Blimp-1 reporter mice and human surgical specimens, we have determined that Blimp-1 is expressed in intestinal T cells under homeostatic conditions in both mice and humans.
Our Chromatin immunoprecipitation (ChIP) studies indicated that Blimp-1 can function as direct repressor of several cytokine genes, including IL-17. We are currently using single cell PCR, RNA-seq and ChIP-seq approaches to identify other target genes and pathways regulated by Blimp-1 in T cells.
Transcriptional regulation of tissue-resident immune cell function
Blimp1’s role in tissue-resident myeloid cells.
A second area of research of our laboratory is the identification of new pathways regulating T cell responses at mucosal surfaces. Mucosal surfaces such as those found in the upper respiratory, urogenital and gastrointestinal tracts function as barriers to infectious microbial diseases, while carrying on many other functions to promote human health. These tissues are enriched in T lymphocytes, which play key roles in protective responses against pathogens but can also be major effectors in deleterious inflammatory responses. Thus, T cell activation and function at the mucosal sites needs to be tightly controlled to assure protection against infection while avoiding chronic inflammation. This task is further complicated by the presence of a complex indigenous flora of microorganisms (including bacteria, fungi and viruses) that inhabits mucosal sites. Despite the broad understanding of T cell activation processes, the regulatory molecular mechanisms controlling T cell responses at the dynamic mucosal sites are not fully understood. We are interested in comparing the behavior of T cells under homeostatic and chronic inflammatory conditions in mucosal sites such as the lung and intestines to identify such regulatory mechanisms.
Research in the Martins laboratory is funded by the NIAD-NIH and the F. Widjaja Foundation.
Mechanisms underlying the generation and maintenance of CD4+ T cell memory responses
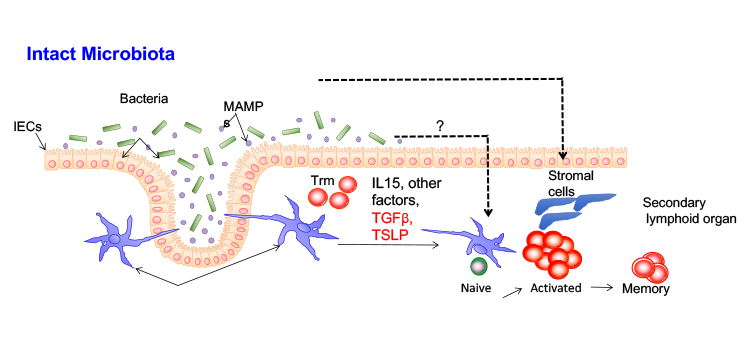
The requirements for the development of protective T cell responses against Staphylococcus aureus.
Regulation of T cell function by the intestinal commensal microbiota.
Contact the Martins Lab
110 N. George Burns Road
Davis Building, Room 4094C
Los Angeles, CA 90048
