Research Areas
Targeting Glioma Stem Cells
Increasing evidence indicates that within malignant gliomas is a small subpopulation of cells, glioma stem cells (GSCs), that have similar properties to normal neural stem cells with genotypes and phenotypes that recapitulate the tumor found within patients with malignant gliomas. The differences between the conventional glioma cell lines and those of GSCs allow us to explore new avenues in gliomagenesis. Understanding GSCs may represent a paradigm shift in not only our understanding of how brain cancer occurs, but also how we model and ultimately treat this disease. The Yu Lab is determining treatment strategies through which we can exploit GSCs relative to conventional glioma cell lines for more appropriate targeted therapy and improved patient outcome.
Dendritic Cell Vaccine Targeting Glioblastoma Cancer Stem Cells
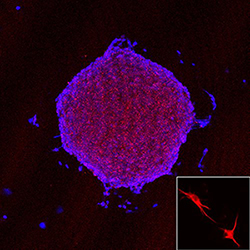
Our translational research focuses on the design of a dendritic cell (DC) vaccine targeting glioblastoma (GBM) cancer stem cells (CSC), which are believed to be the root of the cancer. CD133 is a cell surface protein expressed in the population of GBM tumor cells representing CSC. This raises a possibility that CD133 could serve as a potential target of cytotoxic T cells (CTLs) in GBM CSC immunotherapy. Two potential HLA-A*02-restricted CD133 epitopes, CD133-405 and CD133-753, showed strong binding to HLA-A*0201 molecules. Yu Lab in vitro studies of immunogenicity generated peptide-specific CD8+ cytotoxic T cells from normal donors by using autologous monocyte-derived dendritic cells (MoDC) pulsed with CD133-405 or CD133-753 peptide. These CTLs recognized CD133 expressing HLA-A*0201+ GBM CSCs and were confirmed by interferon-gamma ELISPOT assay. More importantly, the killing assay shows that these CTLs can specifically lyse CD133+ HLA-A*0201+ GBM CSCs without killing the normal bone marrow cells because of lower level human leukocyte antigen expression compared with CSCs. This study has been approved by the U.S. Food and Drug Administration for a Phase I clinical trial.
Chemokine CXC Receptor 4–Mediated Glioma Tumor Tracking by Bone Marrow–Derived Neural Progenitor/Stem Cells
Malignant gliomas manifest frequent tumor recurrence after surgical resection and/or other treatment because of their nature of invasiveness and dissemination. The recognized brain tumor–tracking property of neural progenitor/stem cells opened the possibility of targeting malignant brain tumors using neural progenitor/stem cells. The Yu Lab and others have previously shown that fetal neural progenitor/stem cells can be used to deliver therapeutic molecules to brain tumors. Our recent work has further shown that gene delivery by bone marrow–derived neural progenitor/stem cells achieves therapeutic effects in a glioma model. In this study, members of the Yu Lab isolate and characterize bone marrow–derived neural progenitor/stem cells, which also express the chemokine receptor chemokine CXC receptor 4 (CXCR4). The Yu Laboratory shows that CXCR4 is required for their chemotaxis and extracellular matrix invasion against a gradient of glioma soluble factors. Furthermore, β-galactosidase–labeled bone marrow–derived neural progenitor/stem cells implanted in the contralateral side of the brain were shown to track gliomas as early as day one and increased through days three and seven. Intracranial glioma tracking by bone marrow–derived neural progenitor/stem cells is significantly inhibited by preincubation of bone marrow–derived neural progenitor/stem cells with a blocking anti-CXCR4 antibody, suggesting a CXCR4-dependent tracking mechanism. Glioma tracking bone marrow–derived neural progenitor/stem cells were found to express progenitor/stem cell markers, as well as CXCR4. Although bromodeoxyuridine incorporation assays and proliferating antigen staining indicated that tumor tracking bone marrow–derived neural progenitor/stem cells were mostly nonproliferating, these cells survive in the local tumor environment with little apoptosis. Elucidating the molecular mechanism of brain tumor tracking by adult source stem cells may provide a basis for the development of future targeted therapy for malignant brain tumors. The Yu Lab is presently studying the mechanism of tropism toward stroke to enable the use of bone marrow–derived neural stem cells in restorative therapies for stroke and neurodegenerative disorders.
Nanoplatform Technology to Deliver Drugs Through the Blood-Brain Barrier
Glioblastoma is the most common and aggressive type of malignant primary brain tumor in adults. Despite advances in neurosurgical intervention, radiation therapy, and chemotherapy, the median survival for glioblastoma remains fewer than 15 months after diagnosis. Tumors recur usually within 6 to 12 months of chemoradiation therapy. The treatment of glioblastomas is hampered by the difficulty of delivering therapeutics at efficacious levels through the blood-brain barrier (BBB) to the site of tumors. The BBB is a tightly regulated interface between the blood and brain tissues formed by brain microvascular endothelial cells. The BBB prevents free diffusion of most foreign molecules, including therapeutic agents, except for those that are small, uncharged and lipid-soluble. This is the major obstacle for drug delivery into the brain. However, integrity of the BBB is severely compromised by many diseases in the brain, including brain tumors, neurodegenerative diseases, and traumatic brain injury (TBI). The Yu Laboratory has shown that the nanoprodrug can be delivered through the BBB and highly selectively accumulated in the sites of traumatic brain injury and intracranial brain tumor. The selective accumulation is attributed to the abnormal BBB common in TBI and brain tumor.
Contact the Yu Lab
127 S. San Vicente Blvd.
Pavilion, Suite A-6215
Los Angeles, CA 90048
