Research Areas
Dr. Seki’s laboratory is focused on the study of Inflammation and Extracellular Matrix Regulation in Liver Disease—Liver Fibrosis, Alcohol-associated Liver Disease (ALD), Nonalcoholic Fatty Liver Disease (NAFLD) and Liver Malignancy.
Liver fibrosis is a consequence of chronic liver diseases, such as hepatitis B and C infections, autoimmune hepatitis, nonalcoholic steatohepatitis (NASH) and alcohol-associated liver disease (ALD). Liver cirrhosis, the end stage of hepatic fibrosis, is associated with hepatocellular dysfunction, hepatic failure, portal hypertension and hepatocellular carcinoma. Currently, there is no curative treatment for liver fibrosis, and liver transplantation is the only effective therapy. However, supply of donor livers is always insufficient for all patients that require liver transplantation. In order to develop novel effective therapies for liver fibrosis, we must identify the effective molecular targets. The mission of our research group is to study the molecular mechanism underlying liver fibrosis.
Currently, Dr. Seki’s Laboratory is focusing on three major research topics:
Toll-like receptor signaling in chronic liver disease (liver fibrosis, alcoholic and non-alcoholic steatohepatitis, and hepatocellular carcinoma)
Dr. Seki has reported Toll-like receptor 4 (TLR4), an innate immune receptor for LPS, expressed on hepatic stellate cells (HSCs) and, for the first time, intestine-derived LPS play a central role in HSC activation and liver fibrosis development. His research team also determined that TLR2 and TLR9 contribute to the development of NASH fibrosis. His recent report further demonstrated that macrophage autophagy controls the activation of TLR4 signaling in the development of ALD. Currently, our team studies to develop new therapies for ALD and NASH by targeting TLR signaling.
Role of ECM, hyaluronan, in the progression of chronic liver disease
Our recent study has identified that extracellular matrix (ECM) hyaluronan (HA), used for a biomarker for liver cirrhosis, is the inducer of HSC activation and the cause of liver fibrosis. This study has also proposed a new therapeutic approach for liver fibrosis by targeting HA. Our current research studies more precise mechanisms of HA biosynthesis and catabolism, and HA-induced signaling in liver fibrosis.
Role of underlying liver disease (NAFLD and ALD) in liver metastasis from colorectal, pancreatic and prostate cancer
The large patient cohort reported fatty liver is associated with colorectal cancer liver metastasis. Our recent study has reported that M2 type tumor-associated macrophages and IL-1 are the promoter of colorectal cancer liver metastasis in NAFLD livers. Our ongoing study investigates whether factors secreted from lipid-stored hepatocytes and cancer-associated fibroblasts contribute to enhanced liver metastasis in NAFLD and ALD.
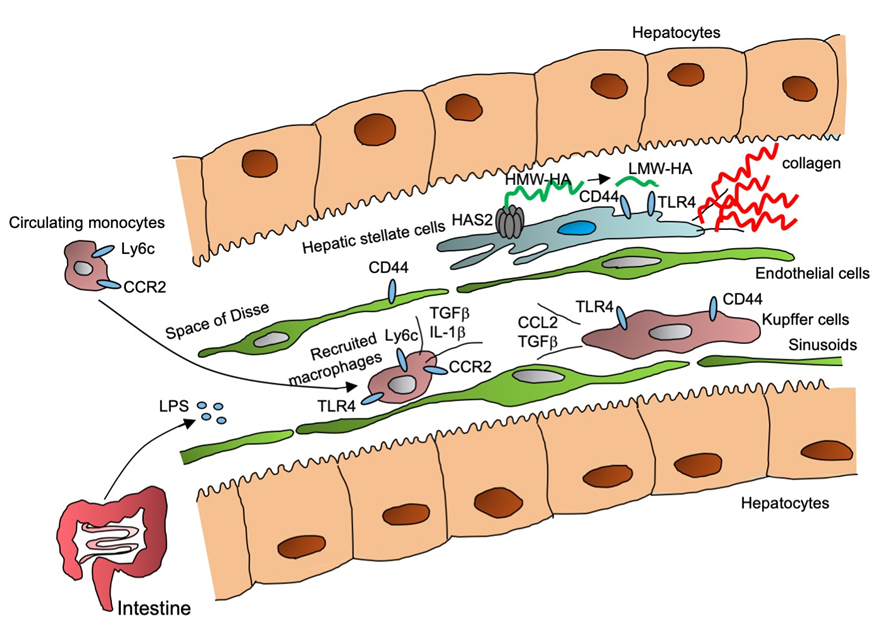
Liver Macrophages and Hepatic Stellate Cells Promote Liver Fibrosis.
Kupffer cells, liver resident macrophages, and recruited macrophages respond to intestine-derived LPS through TLR4 and produce inflammatory cytokines and TGF-ß production. TGF-ß then activates hepatic stellate cells (HSCs), a precursor of hepatic myofibroblasts. Activated HSCs produce hyaluronan (HA), through HA synthesis 2 (HAS2), which enhances the production of collagens, promoting liver fibrosis.
Contact the Seki Lab
8700 Beverly Blvd.
Davis Building, Rooms 2099, 2029
Los Angeles, CA 90048
