Technology & Innovations
Technology Ventures protects and supports the commercialization of discoveries and technologies. Through its activities, Technology Ventures facilitates promising inventions to improve the quality of life for patients around the world.
By the Numbers
Technology Ventures Revenue*
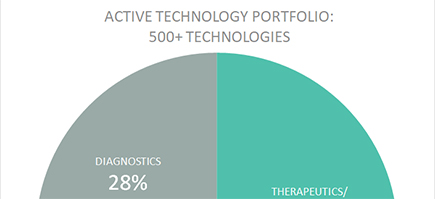
ACTIVE TECHNOLOGIES**
PATENTS ISSUED*
INVENTION DISCLOSURES**
*For fiscal year 2024
**As of July 2024
Ways to Work With Us

Do You Have an Invention to Disclose?
The first step to commercialization is the invention disclosure process, which provides details about the technology of your invention.
Technology Portfolio
Browse our portfolio of technologies available for licensing.

Technology Ventures Team
The team consists of experts in intellectual property portfolio management and licensing, legal affairs and finance.
An Innovation Journey
More than 15 years ago, Mark Pimentel, MD, had a hunch that an antibiotic might alleviate the suffering of his patients with irritable bowel syndrome (IBS).
Resources

Browse our latest report to learn more about our missions and success stories.
Helping inventions and discoveries reach their full potential.
Commercializing valuable diagnostic technologies from Cedars-Sinai and our partners.
(External Link)
Helping entrepreneurs bring their innovative technology products to market.
(External Link)
Dedicated to the development of novel drugs and devices to diagnose and treat patients.
Applying AI to the measurement of parameters critical to understanding the human heart.
(External Link)
Generating world-class biomaterials and conducting groundbreaking research.
Have Questions or Need Help?
Contact us if you have questions or wish to learn more about Technology & Innovations at Cedars-Sinai.


