Research Areas
The neurovascular unit plays a central role in numerous cerebral ischemic and degenerative disorders including acute stroke, chronic cerebral hypoxemia, dementia and Alzheimer’s disease. Therefore, processes involved in the maintenance, remodeling and repair of the cerebral vessels are central hubs for numerous disorders and their potential treatment. The Gonzalez Neurovascular Laboratory has been working in concert with clinical and translational research efforts to study the process of angiogenesis in individuals with cerebrovascular steno-occlusive disorders, such as moyamoya disease and intracranial atherosclerosis (ICAS).
Cerebral Steno-Occlusive Disorders and Angiogenesis
Intracranial atherosclerosis is one of the most common causes of stroke worldwide. It accounts for at least 10% of all strokes in the United States and as much as 67% in countries with predominantly Asian, Hispanic and Black populations. The prognosis for ICAD-caused strokes is worse than for strokes with other etiologies, with an annual rate of recurrent stroke and death of 15% despite intensive medical management, and as high as 35% in certain populations. Recent randomized controlled clinical trials have shown that angioplasty with stenting and bypass surgery fail to improve outcomes in patients with ICAD.
The lab has extensive capability with extensive variability of clinical and imaging patient data that allows interpretation of molecular findings from a clinically applicable perspective. We have conducted extensive analyses on the angiogenic profiles and circulating cytokines of patients with ICAD undergoing medical management and undergoing surgical interventions for revascularization. The profiles that failed medical management are characterized by a predominance of the antiangiogenic factors endostatin, angiostatin and VEGFR1, as well as other growth factors previously identified with high risk of stroke, such as hepatocyte growth factor (HGF).
The figures below schematically represent the components of the eigenvector principal component profile associated with failure of medical management and poor neovascularization after synangiosis surgery.
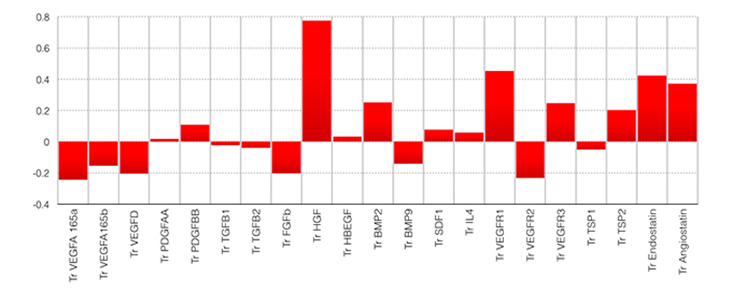
Figure 1. Angiogenic profile (AP4) significantly associated with failure of intensive medical management in patients with ICAD. Notice the predominant components of antiangiogenic factors endostatin, angiostatin, VEGFR1 and the stroke risk predictor HGF. Odds ratio of failure of medical management 7.2 (95%CI= 2.4 – 34.4).
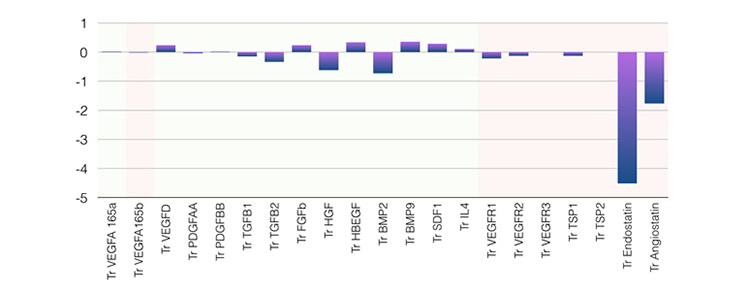
Figure 2. Angiogenic profile significantly (negatively) associated with neovascularization after synangiosis surgery. Notice the predominant components of antiangiogenic factors endostatin and angiostatin (p=0.023).
We have also conducted detailed analysis of the neovascularization of patients treated with synangiosis surgery and characterized the patterns of collateral formation in comparison with native collaterals. Interestingly, our evaluations using branching and tortuosity indexes and local connected fractal dimension show that the vessels formed after encephaloduroarteriosynangiosis (EDAS) follow branching patterns and tortuosity that resemble mature cerebral vessels, contrary to the high tortuosity and low branching patterns of native collaterals (fig. 3)
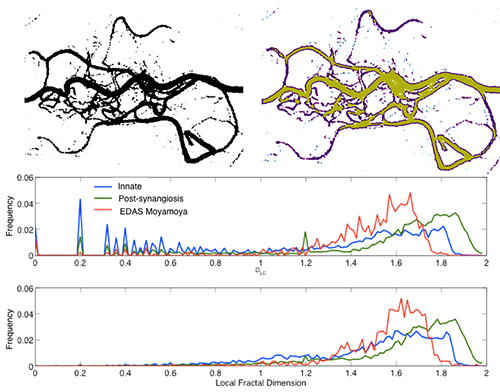
Figure 3. Local connected fractal dimension measured using the FracLac plug-in for ImageJ in an example of post-synangiosis collaterals. There was a significantly higher mean LCFD in the EDAS collaterals compared with the innate collaterals (1.28 +/- 0.1 vs. 1.16 +/- 0.11, P=0.001). The proportion of high connectivity (LCFD ≥ 1.2) in the entire study population was significantly greater in the EDAS collaterals (P=0.001) than in the innate collaterals.
The clinical application of this work has led to the establishment of EDAS as a promising form of indirect revascularization by synangiosis, in which branches of the external carotid artery (ECA) are rerouted intracranially and placed in close contact with the cortical branches of the middle cerebral artery branches, without direct anastomosis. We have been pioneers on this technique and have proven the application of EDAS in adults with moyamoya disease. We recently conducted a National Institutes of Health/National Institute of Neurological Disorders and Stroke-sponsored Phase II clinical trial of EDAS in patients with ICAD. Our study demonstrated a significant reduction in the rates of recurrent strokes and death at one year, as well as an increase in angiographic neovascularization from the ECA branches. EDAS is a promising surgical technique that provides evidence of the safety and effectiveness, supporting further efficacy testing in a Phase III randomized clinical trial, which is currently being evaluated by the NIH for a multicenter nationwide clinical trial of EDAS in ICAD.
Exosomal miRNA Analysis in ICAD
Exosomes are membrane microvesicles that function as important intra and extracellular communication mediators regulating the exchange of proteins and genetic material (i.e., miRNAs). Thus, the Gonzalez Lab has isolated exosomes from the plasma of ICAD patients and analyzed the differential expression of exosomal miRNAs. After reducing the dimensionality of the data using principal component analysis (PCA), we found statistically significant associations of angiogenesis pathways with eight miRNA and four angiogenic factors that were differently expressed between the ICAD patients responsive to and non-responsive to the intensive medical management. Figure 4 shows that the exosomal miRNA and angiogenic factors levels observed in ICAD patients that were non-responder to medical management (red=increased measurement, green=decreased measurement) had a consistent net result of inhibition of angiogenesis (blue=inhibition, orange=activation) affecting the mechanisms of vessel sprouting, branching and proliferation.
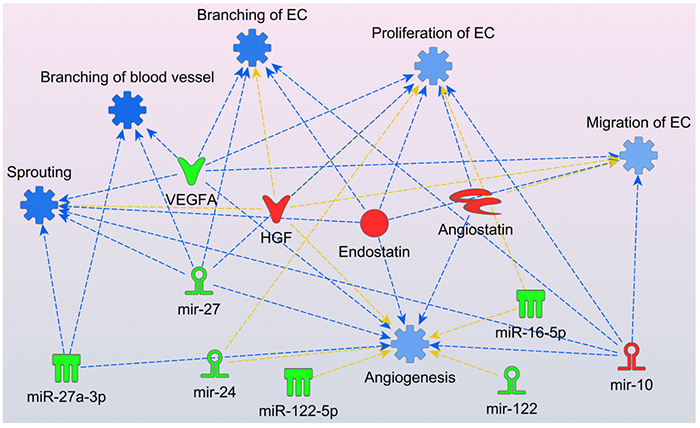
Figure 4. Molecular predictor activity (MAP) from Ingenuity Pathways Analysis (IPA) environment graphical representation of the effects of the observe levels of miRNA and angiogenic factors in ICAD patients failing medical management. Endostatin, angiostatin, HGF and mir-10 were elevated while VEGF-A165a, miR-27, mir-24, miR-122, miR-27-3p, miR-122-5p and miR-16-5p were decreased. Functions and edges marked in blue indicate predicted inhibition. No activation predictions were observed. Yellow arrows show no consistent predicted relationships.
Experimental Model of Synangiosis and Cerebral Ischemia
Having identified candidate angiogenic factors in the treatment of ICAD, we have developed a model of cerebral ischemia in mice by ligation of the distal middle cerebral artery (MCA) (Figure 5).
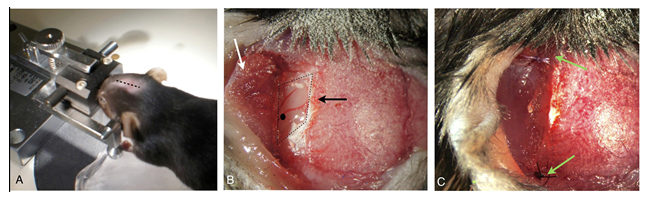
Figure 5. Mice synangiosis model: A) Animal in custom rotating stereotactic frame– midline incision (dotted line). B) Temporal craniectomy (black arrow) and elevated muscle flap (white arrow). Black dot indicates location for MCA ligation. C) Re-approximated muscle flap in contact with subjacent brain (green arrows).
In the model of synangiosis with MCA ischemia, the Gonzalez Lab has demonstrated increased vascular density in the operative side compared with the control side, as shown in Figure 6. On the left panel, endothelial cells were marked with CD31. The surgical side exhibits a conglomerate of increased vascular density at the level of the muscle-brain interphase (red line). Co-staining of endothelial cell marker CD31 (red) and nuclear proliferation marker Ki67 (green) showed significant differences between surgical and control sides (73.0 ± 8.3% vs. 33.6 ± 10.9%, p=0.04), indicating new vessel growth. (We need to add to recent results from the latest experiments with CD31-BrDU co-localization). Structural analysis of the configuration of these new vessels reviewed significantly increased parallelism and reduced branching compared with the native vessels of the control side (Figure 7), representing the morphological features of neovascularization after ischemia-revascularization in the mouse model.
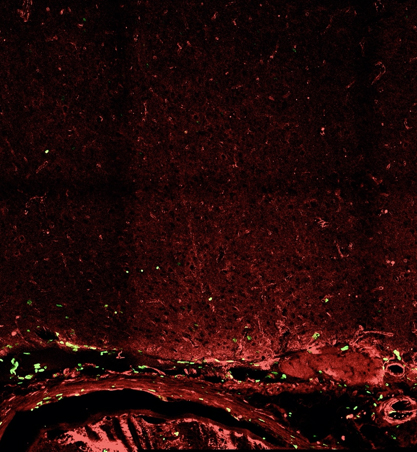
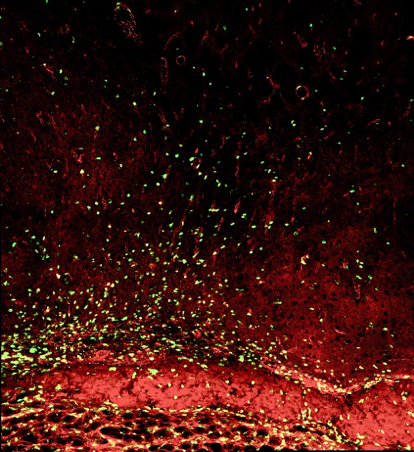
Figure 6. Confocal images of neovascularization after synangiosis surgery showing the significant difference in the number of colocalized endothelial marker CD31 (red), and newly synthesized DNA marker BrdU (green), between experimental (left) and control (right) groups.
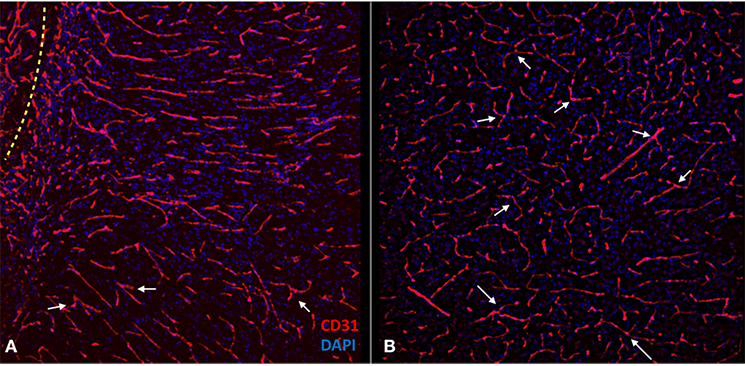
Figure 7. Confocal images of the experimental side (A) and control side (B) depicting the morphological features of the new vessels compared with the native vascular structure of the control side. Notice how the orientation of the vessels is more parallel on the experimental side and perpendicular to the muscle-brain interface (dotted line). The control side has more branching points (arrows) and less parallelism.
Targeting Angiogenesis to Foster Protection from Ischemia
Current efforts in the Gonzalez Laboratory are focused on the evaluation of several strategies to stimulate angiogenesis in the setting of cerebral ischemia, in particular through the development of local agents to facilitate vessel formation after synangiosis surgery.
Contact the Gonzalez Lab
127 S. San Vicente Blvd.
Advanced Health Sciences Pavilion, Suite A6600
Los Angeles, CA 90048