Research Areas
Developing a Personalized Model of Intestinal Fibrosis
Intestinal fibrosis is a common and potentially serious complication of inflammatory bowel disease (IBD) and is caused by the excess deposition of extracellular matrix protein due to chronic inflammation. The mechanisms underpinning fibrosis are complex, involving multiple cells types, cytokines and microbes. There are currently no preventive or therapeutic options and surgical intervention remains the only treatment. Through our collaboration with the Inflammatory Bowel and Immunobiology Research Institute, the Barrett Laboratory can access the Material and Information Resources for Inflammatory and Digestive Diseases Biobank, which contains over 10,000 genotyped lymphoblastoid cell lines (LCLs) that were generated from IBD patients.
The Barrett Lab has previously demonstrated that we can reprogram LCLs to form induced pluripotent stem cells (iPSCs) and direct them to human intestinal organoids (HIOs). By combining this unique bioresource and our innovative technologies, LCLs from IBD patients that have severe fibrosis can be obtained, reprogrammed to IPSCs and directed to form HIOs. This will allow an examination of how cytokines associated with inflammation and fibrosis influence intestinal epithelial and mesenchymal cells from affected IBD patients. Incorporation of these cells into small microengineered Chips will allow a study of the interactions between multiple cell types, microbes and cytokines. The eventual goal of these studies is to unravel the mechanisms underpinning intestinal fibrosis.
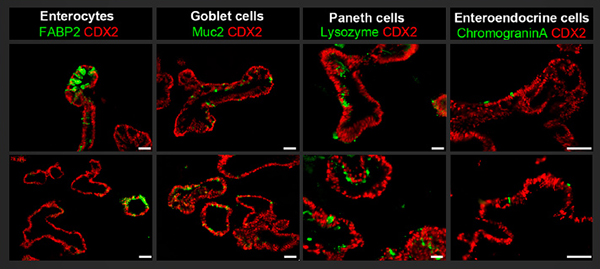
Upper panel shows that induced pluripotent stem cells generated from lymphoblastoid cell lines can be directed to form human intestinal organoids that are immunopositive for the intestinal transcription factor CDX2 and the intestinal epithelial subtypes, including goblet cells (Muc2+), enteroendocrine cells (CGA+), enterocytes (FABP2+) and Paneth cells (Lysozyme+). The lower panel shows similar organoids that were generated from induced pluripotent stem cells that were derived from dermal fibroblasts. Scale bar = 75 μm. (Barrett R, Ornelas L, Yeager N, Mandefro B, Sahabian A, Lenaeus L, Targan SR, Svendsen CN, Sareen D. Stem Cells Transl Med. 2014;3(12):1429-1434.)
Determining Intestinal Epithelial Responses to Cytokines
IBD is believed to be caused by a dysregulated immune response to luminal microbes in genetically defined individuals. However, the role of the intestinal epithelium in the pathogenesis of this disease remains largely undefined. Evidence that epithelial cells are implicated arises from the fact that intestinal permeability is increased in those with IBD.
Numerous factors such as inflammatory cytokines and microbes have been shown to affect permeability yet delineating the precise effect of these components is technically challenging in vivo due to the complex microenvironment. HIO technology allows for intestinal epithelial cells to be maintained for prolonged periods under tightly controlled culturing conditions. This will allow researchers in the Barrett Lab to determine how inflammatory cytokines, away from the confounding influences found in vivo, affect both gene expression and intestinal permeability. Furthermore, this system will allow us to establish how genetic variations, associated with IBD, may influence intestinal epithelial cell function.
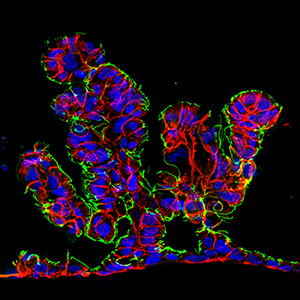
Intestine-Chip: Epithelial cells derived from human intestinal organoids form villous-like structures in response to continuous media flow. CDX2 (red) and E-Cadherin (blue) and ZO1 (green).
Developing New Platforms to Make Human Intestinal Organoids More Amenable for Study
Human intestinal organoids have tremendous potential to allow us to learn about the functioning of the intestinal epithelium but for many assays their complex 3-D structure makes them difficult to utilize. Such challenges include that they are heterogeneous both in shape and size, which may lead to experimental variability and accessing the lumen—crucial for permeability and epithelial-microbial interactions—can be technically challenging. The goal of the Barrett Lab is to make this technology more amenable for study. We have developed methodologies to incorporate intestinal epithelial cells into both transwells and small microengineered Chips. Both allow for consistent numbers of cells to incorporated into each system, allow easy access to the luminal component and for intestinal permeability to be measured. Small microengineered Chip technology additionally allows for prolonged co-culture with microbes and/or other cell types.
Contact the Barrett Lab
127 S. San Vicente Blvd.
Advanced Health Sciences Pavilion, Room 8308
Los Angeles, CA 90048