Research Areas
The focus of the Grivennikov Lab is on the role of inflammation in health and disease with a greater emphasis on the role of inflammation within the tumor microenvironment. Historically, our research centers on the role of cytokines—key regulators of inflammatory responses and tumorigenesis—and cell-type specificity of cytokine signaling. In addition, as microbes are key inducers of inflammatory responses and newly identified regulators in cancer and anti-cancer therapy, we are interested in uncovering how microbes, cytokines and inflammation conspire to promote cancer.
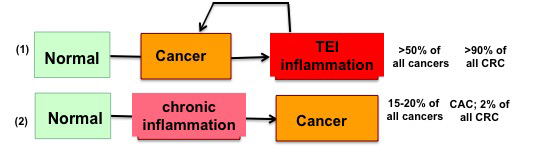
Tumor-Elicited Inflammation
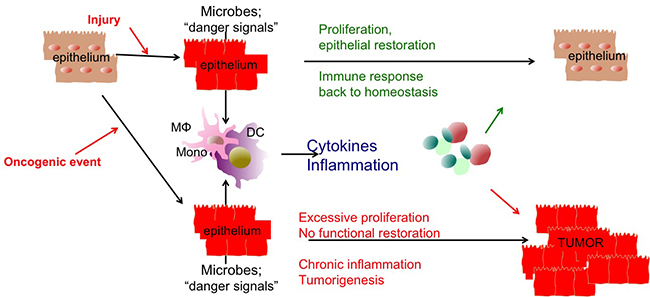
The Grivennikov Lab helped to develop a concept of tumor-elicited/associated inflammation (TEI) as a phenomenon observed in most initially noninflammatory tumors. In such tumors, nevertheless, intrinsic inflammation is induced and inflammatory pathways are activated through a variety of mechanisms. In sporadic colon cancer (CRC), we found that cytokine interleukin-23 (IL-23) regulates the expression of other inflammatory cytokines, and recruitment of immune cells into tumors, therefore, constituting a master regulator of TEI. We determined that TEI is induced early in CRC, arising from deterioration of epithelial barrier and tumor-specific translocation of microbes. We propose that similar TEI mechanisms are likely to be present in various types of cancers. We therefore currently test how TEI develops at the tumor site after tumor initiation, and how TEI is regulated in the tumor microenvironment of CRC and other cancers and determine how cytokines, sterile-inflammation, oncogene-induced inflammation or microbiota-induced inflammation works. Below are main research areas in the Grivennikov Lab, which are constantly shaped by the outcomes of ongoing research, interests and scientific aspirations of research team members.
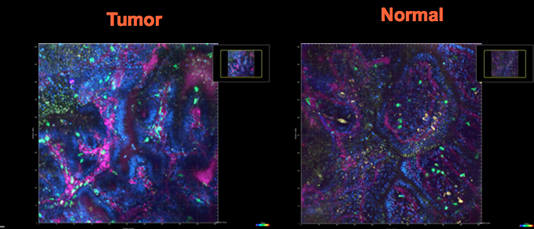
Role of Cytokine Pathways and Inflammation in Inflammatory Bowel Disease and Colon Cancer
The lab studies how cytokine signaling affects the development of inflammatory bowel disease (IBD) and colitis-associated (CAC) and spontaneous (CRC) colorectal cancers. As receptors for cytokines are expressed by cancer and epithelial cells, cytokines are ideal candidates for multicellular cross-talk between immune/inflammatory and cancer cells. Indeed, cytokines not only promote inflammation, but also serve as growth and protective factors for epithelial cells in acute and chronic inflammation and tumorigenesis. In addition, cytokines modulate “inflammatory tone” by differentially regulating stromal and immune cells. Here, we use in vivo conditional genetic mouse models to dissect detrimental and protective roles of cell-type-specific cytokine signaling in chronic inflammation and cancer.
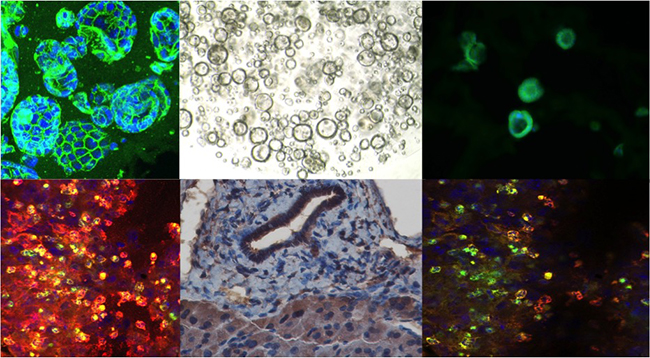
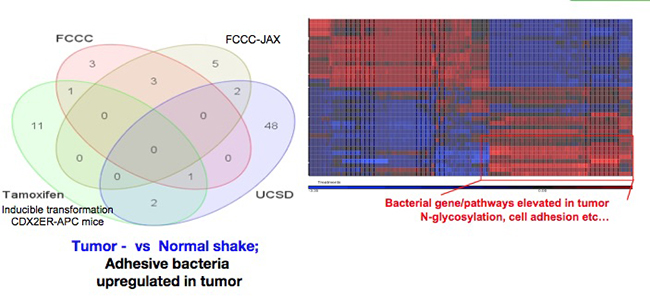
Role of Functional Microbiota in Induction of Inflammation, Tumorigenesis and Cancer Metastasis
As the microbiome has recently been shown to be a critical regulator of many pathophysiological processes including obesity, autoimmune diseases and cancer, our lab and others demonstrated that antibiotic-treated or “germ-free” mice have reduced IL-23/IL-17 expression and TEI, and reduced CRC tumor burden. However, the taxonomical and functional identity of the microbes promoting CRC and TEI, are currently unknown.
We are interested in bacteria and microbiota adjacent to inflamed and cancerous tissues, especially those that adhere to tumors or can breach through epithelial barriers and travel to distant sites. We extensively use complex mouse models for colon, pancreatic and other cancers along with genetic or pharmacological targeting of host pathways. We characterize such cancer-relevant bacteria through multiple approaches including 16S RNA and metagenomic sequencing, microbiota addition or depletion, as well as metabolomic characterization and inactivation of host pathways of bacteria sensing to determine: whether and how bacteria or bacterial products affect local, systemic or distal inflammatory responses; how inflammation-dependent and independent action of microbiota affect tumor initiation, growth, progression, metastasis and therapy resistance; and whether the role of microbiota and underlying mechanisms are similar or different for colon or pancreatic cancers, and for primary or metastatic cancers.
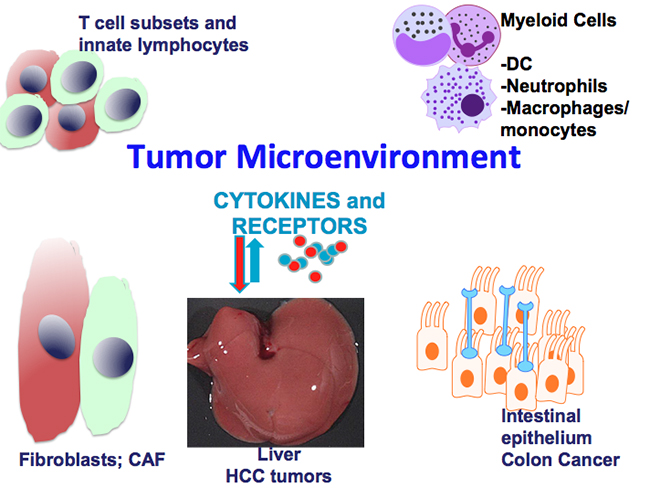
Role of Tumor Microenvironment in Progression of Colon and Pancreatic Cancers—Tumor Promotion and Sensitivity to Immunotherapies
We are interested how signaling by key cytokines (IL-17, IL-1, IFNg) in different cellular components of complex tumor microenvironments shape tumorigenesis, progression and metastasis of colon and pancreatic cancers. We are focused on cytokine signaling in myeloid cells, subsets of fibroblasts and epithelia/cancer cells and use a variety of genetic and single-cell-based approaches to determine how cytokine signaling regulates cell activation and cellular plasticity in cancer. Finally, we aim to uncover how inflammatory signaling within the tumor microenvironment can be targeted to alleviate pro-tumor effects and sensitize cancers to novel immunotherapeutic approaches.
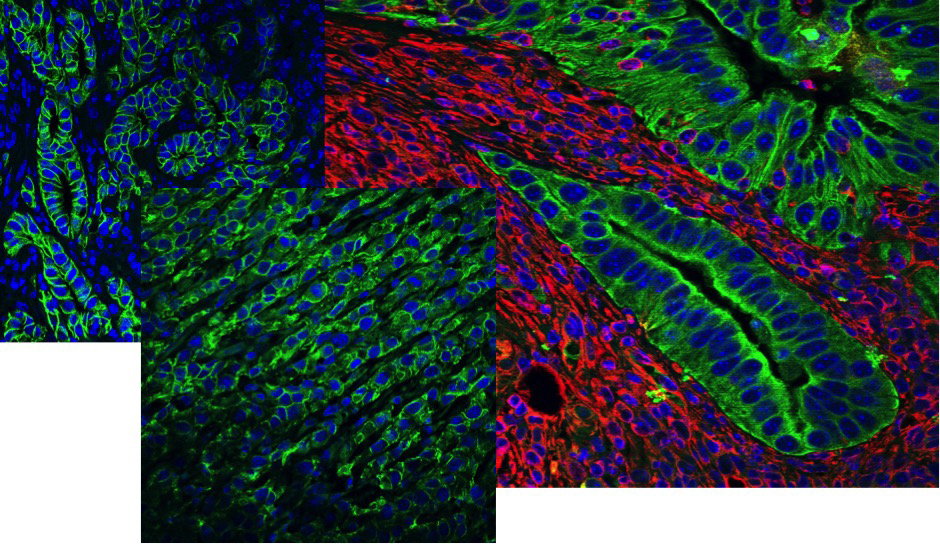
Inflammation and Cytokines in Tissue Injury and Regeneration
We study how microbial and inflammatory signals contribute to the development of tissue injury or tissue healing/regeneration in the intestine and liver using in vivo models.
Contact the Grivennikov Lab
8700 Beverly Blvd.
Davis Building, Suites 2094D & E
Los Angeles, CA 90048