Research Areas
Research in the Hogaboam Laboratory addresses two major themes related to chronic pulmonary disease.
The first encompasses the cellular and molecular immune mechanisms that regulate the pulmonary growth and persistence of fungal (summarized in Figure 1) and viral signals in a number of experimental murine models of allergy, hypersensitivity and asthma. Our data to date has revealed that key immune cells such as myeloid cell progenitors, macrophages and dendritic cells bridge the pulmonary innate and adaptive immune responses to these signals. We are presently pursuing several therapeutic interventions with industry collaborators.
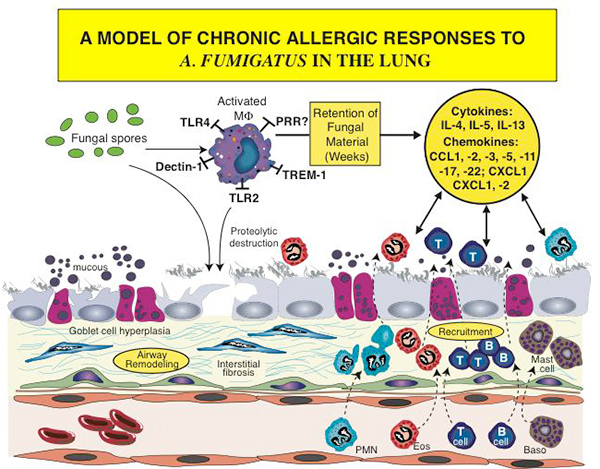
Figure 1. An outline of the chronic responses of airway remodeling and cell recruitment, which are initiated and sustained in the allergic airway due to exposure to fungi such as Aspergillus fumigatus. Both immune cells (i.e., macrophages, dendritic cells, T cells, neutrophils (PMN), eosinophils (eos), mast cells and basophils (baso) and nonimmune cells (i.e., epithelial cells, goblet cells, fibroblasts and endothelial cells) play prominent roles in the generation of and response to soluble protein factors such as cytokines and chemokines.
Second, and more translational, we explore a grouping of clinical diseases collectively known as idiopathic interstitial pneumonias (IIPs). IIPs are characterized by a lethal fibrotic response due to an exuberant fibroproliferative response by collagen-producing cells in the lung. The mechanism leading to aberrant fibroproliferation in these diseases is an active area of investigation, and we are presently testing the hypothesis that a "misguided" innate immune response is a major cause for destructive fibrotic responses (Figure 2). Using human lung diagnostic biopsy tissues and isolated human cell populations (e.g., stromal and stem/progenitor-like), the Hogaboam Lab is using large-scale genomic, proteomic and novel animal model approaches to explore the etiopathogenesis of these diseases and, more importantly, identify novel therapeutic targets in IIPs.
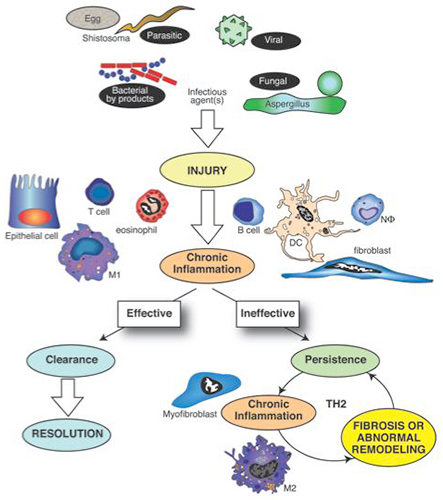
Figure 2. Pathogens such as bacteria, viruses, fungi and multicellular parasites represent major tissue injury signals. The host response, mediated by the innate immune system, is intricately directed toward recognizing pathogen-associated molecular pathogens (PAMPs) through a myriad of pattern-recognition receptors (PRRs). A dominant Th1 cytokine response (characterized by interferon gamma) normally characterizes an effective immune response against most pathogens, except most multicellular and extracellular parasites. Should pathogens and their byproducts be effectively cleared, the affected tissue often heals appropriately and the inflammatory process resolves. However, pathogens use various survival strategies to avoid elimination and this leads to their persistence or the persistence of their byproducts in the host. The cytokine pattern associated with the ineffective response is often skewed toward the Th2 cytokine pattern (characterized by IL-4 and IL-13). The resulting fibrotic response appears to be a consequence of the actions of unique cells such as the ECM-component synthesizing myofibroblast and the alternatively activated macrophage (M2 macrophage). The fibrotic response in turn might facilitate the persistence of pathogens and their byproducts, thereby allowing this vicious cycle to continue.
Contact the Hogaboam Lab
127 S. San Vicente Blvd.
Pavilion, 9108
Los Angeles, CA 90048