Research Areas
Koronyo-Hamaoui's research is unraveling the role of innate immune cells, particularly microglia and peripheral monocytes and macrophages, in CNS repair and regeneration. Her groundbreaking work extends to the development of novel immunomodulation and epigenetic-based treatments for Alzheimer's disease (AD). With a focus on the neurosensory retina, she explores pathological signatures of AD, aiming for early screening and monitoring response to therapy. As a pioneer, her team identified the core pathological hallmarks of AD, along with inflammatory, vascular and neurodegenerative biomarkers in the retinas of AD patients, including those at the earliest stages of functional impairment.
The retina-brain connections are being investigated to define what retinal changes mean for brain pathology and cognitive performance. Her group developed a breakthrough noninvasive approach for detecting retinal Aβ deposits using high-resolution optical imaging, marking a significant stride towards early, more accurate and accessible AD imaging. The team is dedicated to their quest for establishing transformative diagnostic and intervention strategies for AD—a call to action to collectively end Alzheimer's disease.
Retinal Pathology in Alzheimer's Disease (AD)
Study of pathological changes in the retina of Alzheimer’s disease patients and animal models; investigation of early abnormalities that may appear in the retina prior to the brain and their correlation with brain pathology
The presence of Aβ deposition in postmortem retinas of definitive Alzheimer’s patients and in early-stage cases has been identified for the first time by the Koronyo-Hamaoui Laboratory. In addition, the team developed a noninvasive retinal imaging approach for detection of plaques in live animal models. Administration of curcumin, a natural and safe fluorophore that binds to Aβ, allows detection of retinal plaques in-vivo by very sensitive, noninvasive optical imaging. Results from these studies indicate that the hallmark pathology of AD, Aβ plaque, is not restricted to the brain but also exists in the retina. A correlation was found between the reduction of plaque in the brain and in the retina following immunotherapy in transgenic murine models. Currently, the group is seeking to correlate the accumulation of toxic Aβ to other retinal abnormalities and to visual dysfunctions. Methods such as these may help establish new approaches for early, noninvasive, and definitive diagnosis of AD while enhancing ability to monitor progression and responsiveness to therapeutic interventions.
Exploring AD in Human Retina
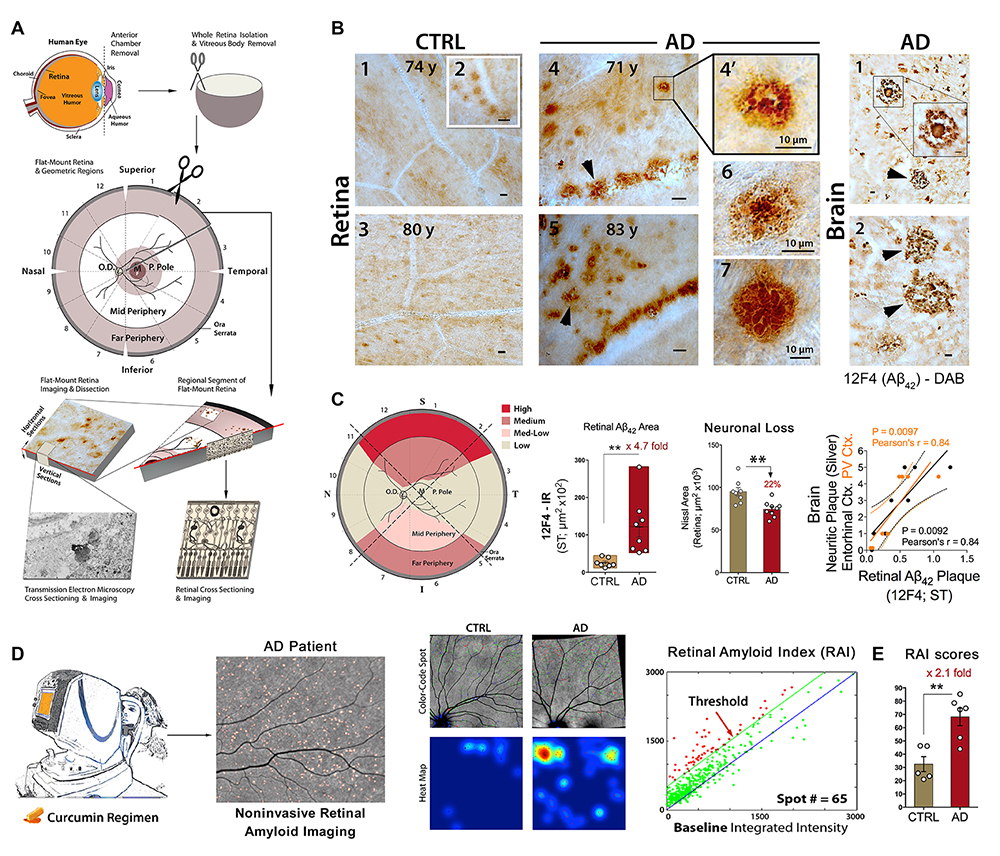
First identification and noninvasive live imaging of retinal Aβ deposits in Alzheimer's disease (AD) patients. A. Schematic diagram of donor eye dissection, whole retina isolation, and tissue processing for histological analysis. B. Representative micrographs from flat-mount retinas and brains of neuropathologically-confirmed AD patients relative to age- and sex-matched cognitively normal controls (CTRLs). C. Left: Qualitative geometric distribution of retinal Aβ deposits in AD patients (S – superior, T –temporal, I – inferior, and N – nasal quadrants). Middle: Quantitative 12F4-immunoreactive (IR) area of Aβ42-containing plaques and quantitative Nissl+ neuronal area in retinas from AD patients vs. matched CTRL. Right: Correlation between 12F4-IR area of Aβ42-containing plaques and brain neuritic plaques. D. Illustration of noninvasive retinal amyloid imaging using Longvida® curcumin and a modified scanning laser ophthalmoscope in human trials, leading to the creation of retinal amyloid index (RAI). E. In a proof-of-concept trial, a significant increase in RAI scores measuring retinal amyloid-plaque burden is found in retinas of AD patients vs. matched CTRLs. Republished with permission of American Society for Clinical Investigation from Koronyo et al. JCI Insight 2017; permission conveyed through Copyright Clearnance Center. Relevant publications: Koronyo-Hamaoui et al. Neuroimage 2011 (2010 online), Koronyo et al. Neurodegener Dis. 2012, La Morgia et al. Annals of Neurology 2016, Hart et al. 2016, Koronyo et al. JCI Insight 2017, Doustar et al. Front Neurol. 2017, Hampel et al., JAD 2018.
Loss of Retinal PDGFRß/Pericytes along with Vascular Amyloidosis in MCI and AD Patients
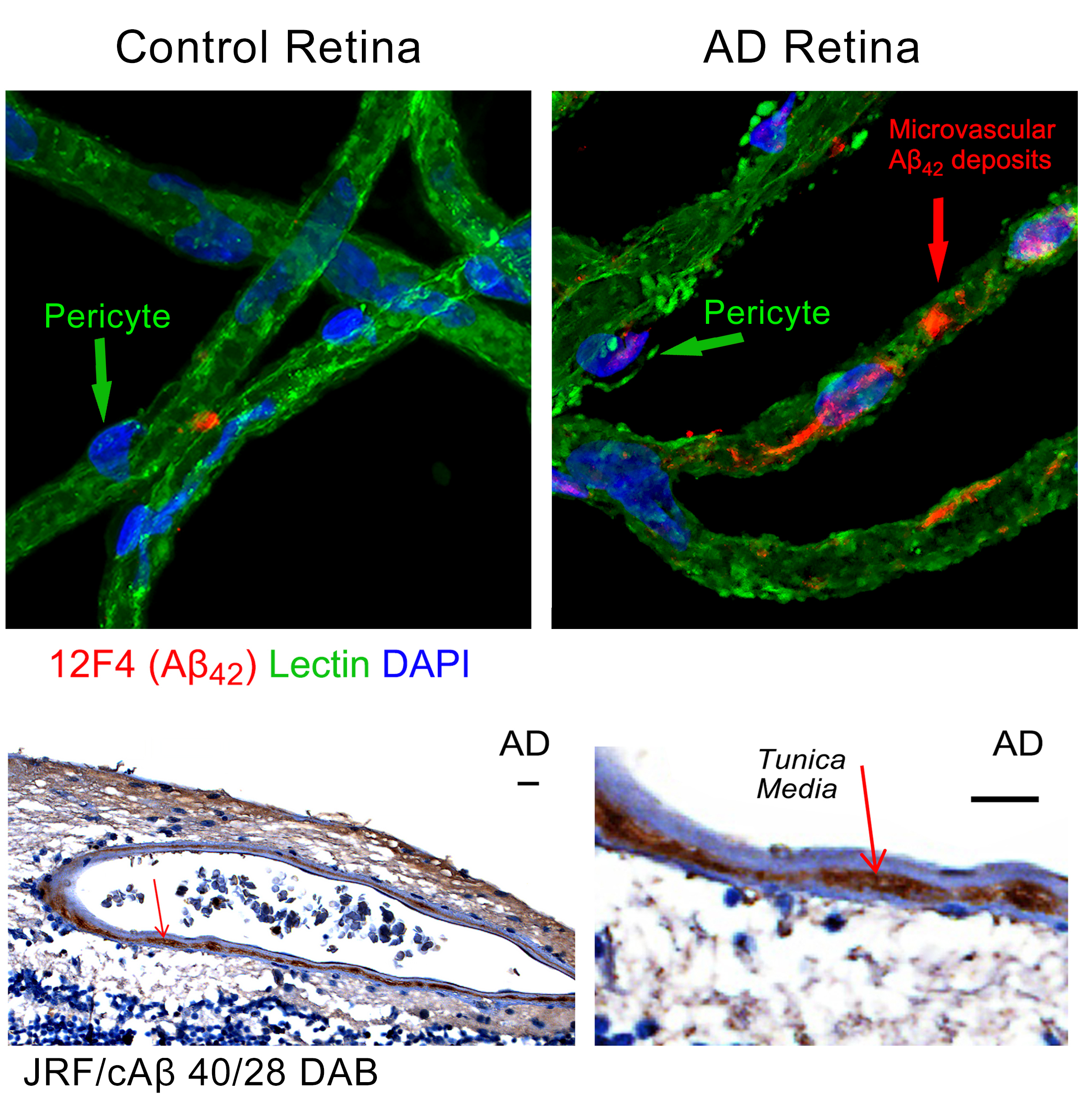
Discovery of early vascular pericyte loss and amyloidosis in the retina of MCI and AD patients. Adopted from Shi et al. Acta Neuropathologica 2020.
Immunomodulation Therapy for AD and the Role of OPN/SPP1
Study of immunological mechanisms of repair and regeneration in the central nervous system in transgenic rodent models; development of effective immune-modulation therapies to prevent, retard and perhaps cure Alzheimer’s disease
The inflammatory responses that are prominently found in the brains of AD patients and tightly associated with Aβ plaques were perceived as merely detrimental contributors to disease progression. In contrast to this traditional notion, converging evidence, including from the Koronyo-Hamaoui group, has shown that well-controlled adaptive and innate immune responses can limit neurotoxicity, clear cellular debris, enhance neuroprotection, and restore brain homeostasis and cognitive function (Figure 2). Koronyo-Hamaoui’s neuroimmunology laboratory investigates immunological mechanisms of central nervous system (CNS) repair and regeneration in attempt to develop effective and risk-free means of harnessing a protective immune response to treat the pathology associated with neurodegenerative disorders, especially AD.
Role of OPN/SPP1 in AD
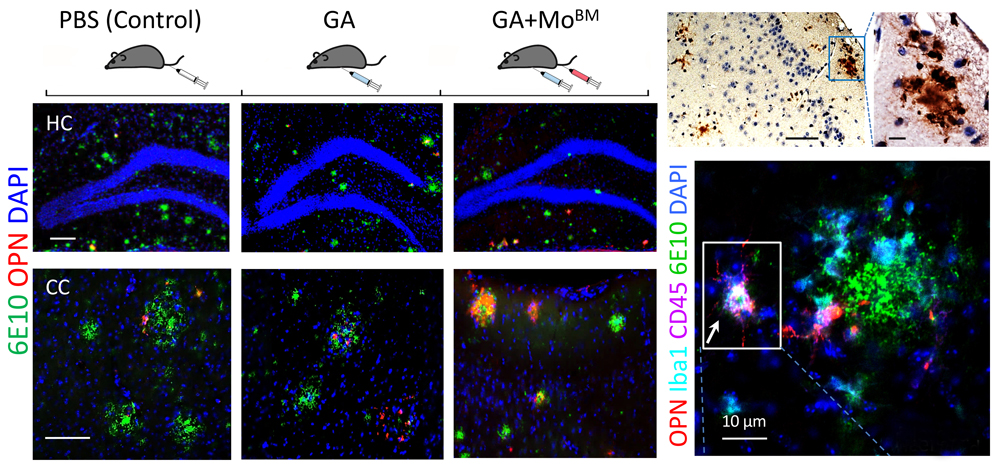
Boosting peripheral monocytes and their immune activation in murine models of AD increases OPN/SPP1 in brain macrophages involved in resisting AD pathology and preserving cognition (Rentsendorj et al. BBI 2018).
Synaptic Preservation by Activated Macrophages
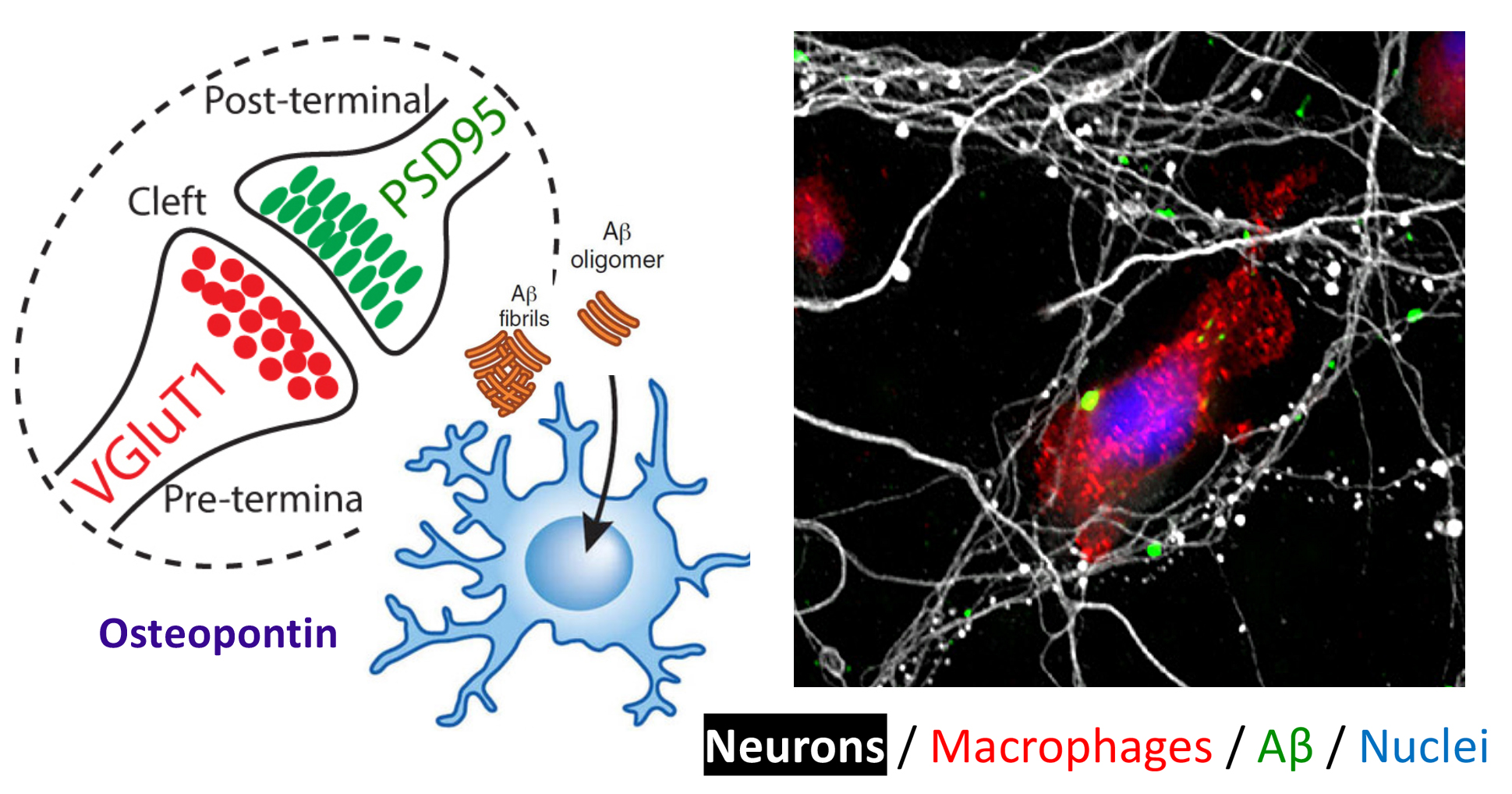
Synaptic preservation against oligomers of amyloid-beta is achieved by activation of bone marrow-derived macrophages. Adopted from Li et al. Frontiers in Immunology 2020.
Therapeutic Role of Monocytes in AD
With the intention of acquiring a more complete understanding of this immune-based approach for AD treatment, the Koronyo-Hamaoui Laboratory experimented with bone marrow-derived monocytes. Upon depletion of bone marrow-derived monocytes in ADtg murine models, an exacerbation of AD-like progression (i.e., more plaques in the brain) was observed. However, following GA or DC-45D immunization, and by enriching the blood with monocyte-derived macrophages or replacing old bone marrow with young wild-type bone marrow-derived monocytes, increased recruitment of blood-borne monocytes was detected in the ADtg mouse brain. Subsequently, there was a reduction in Ab plaques and attenuation of disease progression. Furthermore, the team recognized the subset of bone marrow-derived therapeutic monocytes in AD as inflammatory monocytes. The benefits derived from these inflammatory monocytes are associated with an increased plaque clearance, regulation of inflammation via IL-10, and enhanced catalytic degradation of Ab (i.e., MMP-9). The lab continues to research the activity of these peripheral immune cells in the brain, and their possible impact on mediating tissue-healing processes.
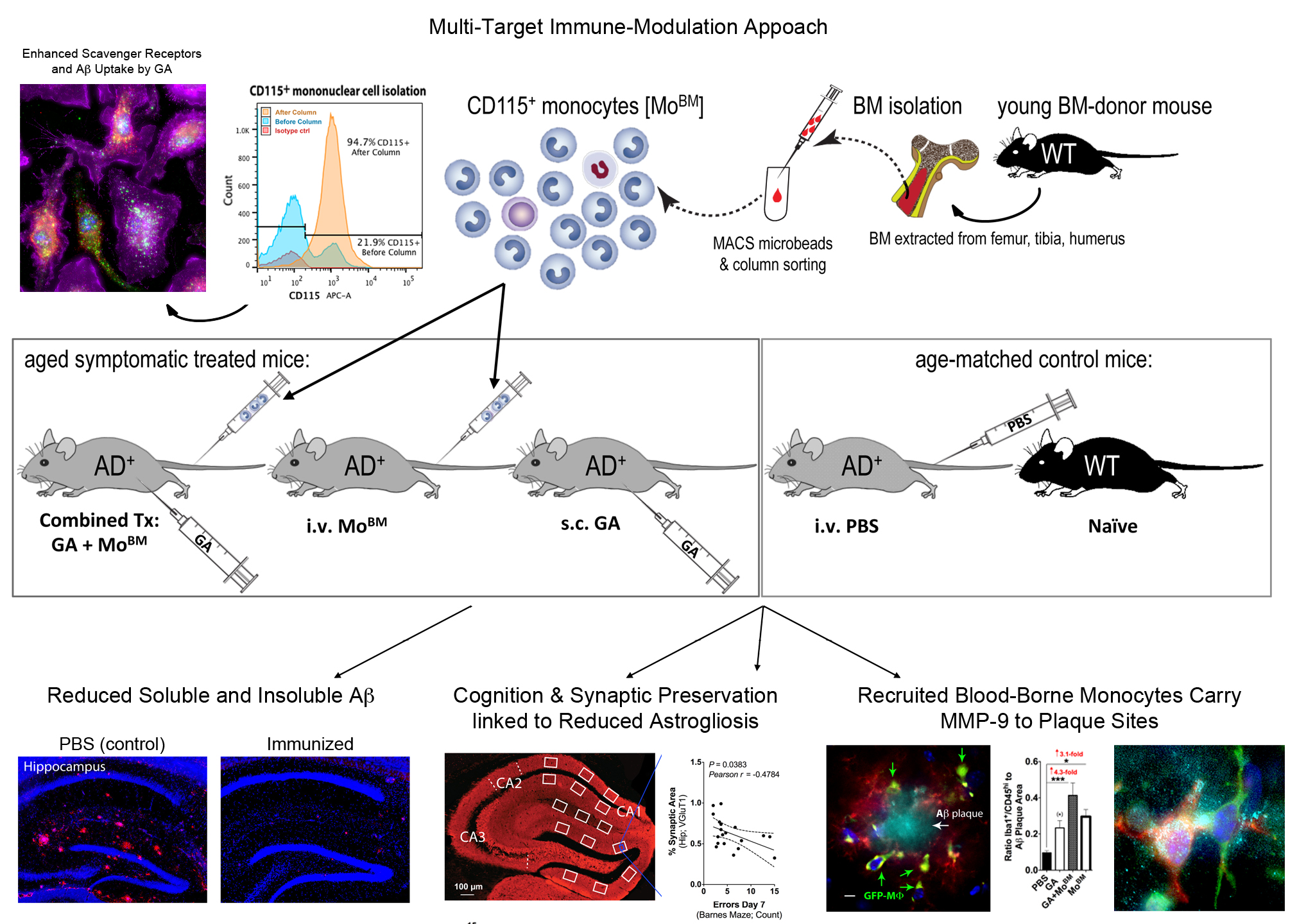
The FDA approved drug Copaxone (glatiramer acetate) can be redirected to resist the pathology and symptoms associated with Alzheimer’s disease (Koronyo et al. Brain 2015, Koronyo-Hamaoui et al. JNC 2009, Zuroff et al. CMLS 2017). Adoptive transfer of bone marrow (BM)-derived monocytes to peripheral blood of AD+ mice produced similar neuroprotective effects (Koronyo et al Brain 2015).
Targeted Overexpression of Angiotensin Converting Enzyme (ACE) in Macrophages
In addition to the results on the therapeutic role of monocytes, a similar investigation is underway to assess the role of genetically enhanced monocytes in AD. ACE is a peptidase capable of cleaving Aβ1-42 peptide into less toxic peptides. In previous studies, ACE was shown to affect monocytic cell behavior, enhancing these innate immune cells’ ability to combat cancer as well as viral and microbial infections. The Koronyo-Hamaoui Laboratory has found that a genetically modified murine model of AD overexpressing ACE results in a remarkable attenuation of disease progression and cognitive preservation. Degradation of cerebral Ab plaques was associated with their enhanced clearance by monocyte-derived macrophages. Further endeavors will identify and clarify the role of ACE overexpression in the brain regarding the progression of AD along with its applications to immune-based therapy specifically involving monocyte function. Results from these studies may positively impact the availability of effective disease-modifying therapies for AD.
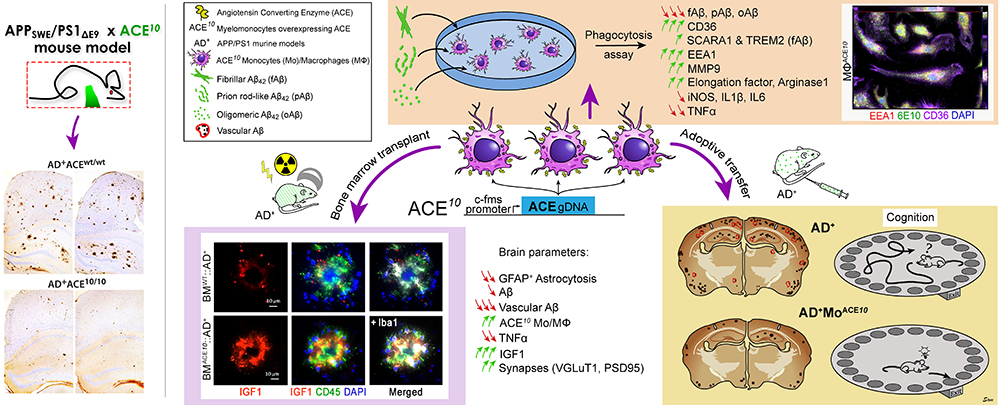
Left panel: Our original report discovered minimal cerebral Aβ pathology in the transgenic APPswe/PS1dE9 mouse model of AD that overexpresses ACE in myelomonocytic cells (ACE10/10), including resident microglia and infiltrating monocytes. Therapeutic effects were dependent on catalytic activity of ACE, an Aβ-degrading enzyme (Bernstein et al. JCI 2014, Koronyo-Hamaoui et al. Curr Hypertens Rep 2014, Bernstein et al. Biol Chem 2014, Koronyo-Hamaoui et al. Brain 2019).
Right panel: In a recent study, we utilized both in vivo and in vitro approaches to reveal the role of transient and targeted overexpression of ACE to BM-derived monocytes in attenuating AD-related pathologies and preventing cognitive decline. We report that ACE-overexpressing macrophages preserved synapses and cognition as well as enhanced resistance to defined pathogenic Aβ forms. This translational immunomodulation approach highlights the potential of peripheral monocytes and ACE to fight AD (Koronyo-Hamaoui et al. Brain 2019, 1-23, doi:10.1093/brain/awz364). Reproduced by permission of Oxford University Press on behalf of the Guarantors of Brain.
T Cell-Based Immunization
The Koronyo-Hamaoui Laboratory was able to demonstrate that immunization with glatiramer acetate (GA) or MOG45D loaded on dendritic cells (DC-45D) resulted in significant attenuation of Alzheimer’s-like pathology and improved cognitive performance in murine models. These preclinical studies have shown that immunization evoked a peripheral immune response involving activity of Th2 cells and recruitment of protective monocyte-derived macrophages to regions containing Aβ plaques in the brain. Infiltrating monocyte-derived macrophages are shown to be highly effective in clearing Aβ plaque and in regulating local inflammation. Hence, both GA and DC-MOG45D immunizations were found to target multiple malfunctions occurring in AD; including regulation of detrimental inflammation (i.e., elevated IL-10, TGF-b1, reduced TNF-a), resolution of scar tissue (CSPG, GLT-1 in astrocytes), secretion of Aβ-degrading enzymes (i.e., MMP-9), as well as supporting synaptogenesis and neurogenesis (i.e., Egr-1, IGF-1).
Transcription factor Egr-1 has been discovered as a mediator of neural survival and neurogenesis in neurodegenerative models
Supplementary search of immune-based mechanisms in the CNS allowed the team to observe restoration and increased formation of newly formed neurons from the brain progenitor pool. In rat models for chronic glaucoma and in mouse models of AD, the lab has unveiled a momentous induction in a newly identified transcription factor (Egr-1) as a result of GA immunization. Egr-1 was correlated with increased neuronal survival in glaucomatous retina, and with reduced brain pathology and increased regeneration and cognitive function in Alzheimer’s models. Nonetheless, the exact mechanism by which GA or MOG45D exhibit neuroprotection is largely unknown. Further research explores other mediators of monocyte recruitment and immune regulation in the transgenic mouse brain.
Immune-CNS Interactions in Neurodegenerative Diseases
Pathological aging of peripheral immune system in ALS, Parkinson’s and Alzheimer’s diseases
Maya Koronyo-Hamaoui, PhD, and Michal Schwartz, PhD, in collaboration have identified key factors involved in a pathological aging of the immune system in the thymus of amyotrophic lateral sclerosis (ALS) mice and human patients. The results from these studies propose that natural killer T cells with decreased hepatic expression of IGF-1 are potential contributors in ALS, and that the liver is a site of a major pathology in this disease. Another study, in collaboration with Dwain Morris-Irvine, PhD, MPH and Christopher Wheeler, PhD, demonstrated that Parkinsonian rodents deficient in T cells perform poorly on behavioral tests and lose selective dopaminergic neurons. These studies may lead to novel immunotherapies and diagnostic biomarkers for Parkinson’s (PD). The combined studies suggest that specific functions of age-related defective T cells can cause AD or PD-like pathology and may contribute to ALS progression, and identifies certain factors as potential targets to counteract neurodegeneration.
Contact the Koronyo-Hamaoui Lab
Cedars-Sinai Maxine Dunitz Neurosurgical Institute
127 S. San Vicente Blvd.
Advanced Health Science Pavilion, Sixth Floor, Suite A6212
Los Angeles, CA 90048