Research Areas
The pituitary is a small endocrine gland that controls vital homeostatic functions. It is called the “master gland” because it directs other organs and endocrine glands, such as the thyroid, the adrenals and the liver to suppress or induce hormone production. Under control of growth factors and transcription factors, pituitary progenitor cells differentiate into five types of hormone-secreting cells: lactotrophs (prolactin secreting), somatotrophs (growth-hormone (GH) secreting), gonadotrophs (follicle-stimulating hormone and luteinizing-hormone secreting), thyrotrophs (thyroid-stimulating hormone secreting) and corticotrophs (adrenocorticotrophic-hormone (ACTH) secreting).
Growth Hormone and Epithelial Cell Proliferation
Vera Chesnokova, PhD, studies the role of GH in the neoplastic transformation of epithelial cells. Environmental insults and aging lead to DNA damage, which, if unrepaired, results in chromosomal instability and tumorigenesis. She found that local non-pituitary GH (npGH) is induced in human colon and human intestinal organoids with age in response to DNA damage, induces cell proliferation, and suppresses epithelial DNA damage repair, resulting in DNA damage accumulation (Chesnokova et al., Cell Rep. 2021). Circulating GH decreases with age, and GH therapy has been advocated by some to sustain lean muscle mass and vigor in aging patients. These results elucidate a mechanism underlying GH-activated epithelial cell transformation and highlight an adverse risk for inappropriate adult GH treatment. As age-associated npGH induction enables a pro-proliferative microenvironment, abrogating npGH signaling could be targeted as anti-aging therapy by impeding DNA damage and age-related pathologies.
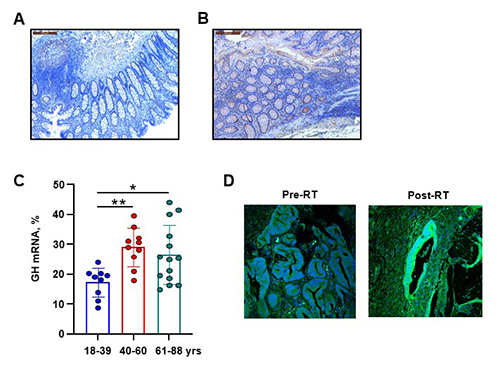
Figure 1. Human colon DNA damage and npGH increase with age. Representative IHC images of GH expression (brown) in human colon specimens derived from (A) 29-year-old and (B) 58-year-old patients. Graph depicts percent of patients expressing γH2AX with IHC score ≥50. Between 5 and 7 fields were analyzed per sample. (C) Graph depicts percent of GH mRNA-positive cells per field; each dot represents one patient. Results were analyzed by ANOVA followed by Tukey’s test to correct for multiple groups. *p<0.05, **p<0.01. (D) GH is induced in response to DNA damage. Human colon adenocarcinoma specimens derived from the same patients before (Pre-RT) and after (Post-RT) DNA-damaging radiotherapy. GH, green; DAPI, blue.
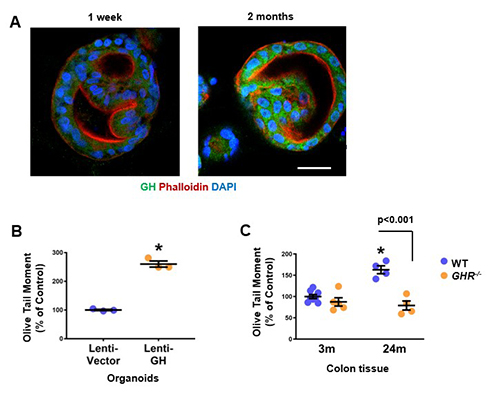
Figure 2. GH suppresses DNA damage repair and enhances DNA damage accumulation. (A) GH induction in aged organoids. Confocal image of human intestinal organoids were cultured for up to 2 months. (B) GH enhances DNA damage accumulation in organoids infected with lenti-GH as compared to control organoids infected with lenti-vector for 30 days. DNA damage was assessed by Olive tail moment. *p<0.05. (C) Abrogated GH signaling reduces DNA damage in aging mouse colon. Olive tail moment in the colon of young (3 months) and old (24 months) WT and GHR-/- mice. *p<0.001 WT old vs both WT and GHR-/- young mice.
Drivers of Somatotroph Adenoma Progression
Anat Ben-Shlomo, MD, studies genomic changes that underlie development and progression of pituitary tumors, particularly GH-secreting somatotroph adenomas that lead to acromegaly. Using whole-exome sequencing and KEGG pathway analysis, she has shown somatic copy number alterations (SCNAs), rather than genetic mutations, are hallmarks of these tumors, and that activating the cAMP signaling pathway increases DNA damage (Ben-Shlomo et al., J Clin Invest. 2020). These findings help explain different phenotypes and treatment responses in patients with acromegaly and provide a basis for further studies linking SCNAs, cAMP signaling and DNA damage in the development of somatotroph adenomas.
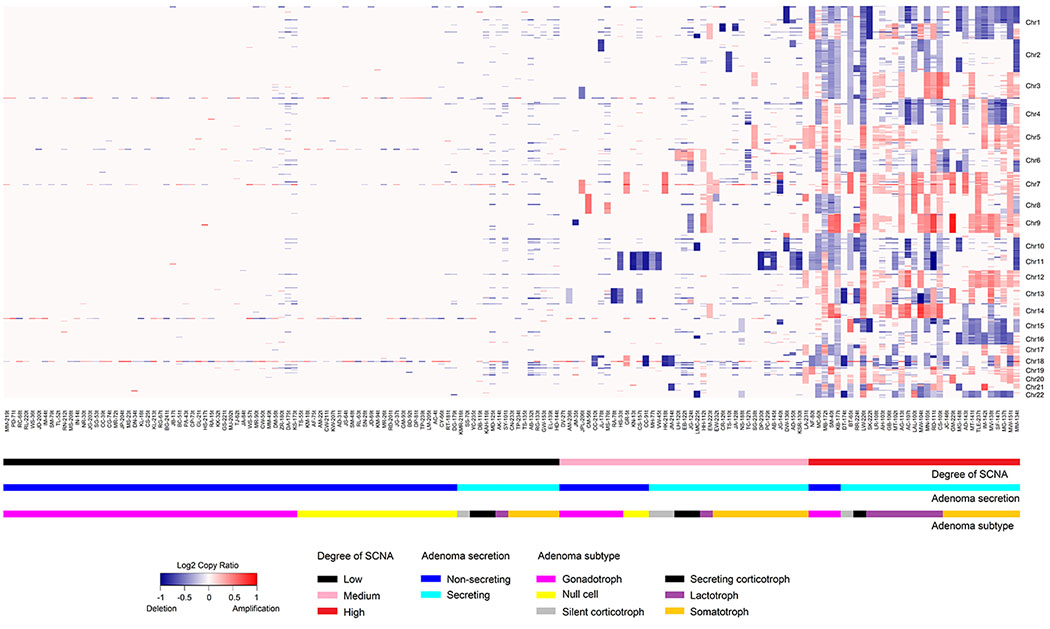
Figure 1. Heatmap of SCNAs obtained from whole-exome sequencing of 159 adenomas depicting SCNA copy ratio as well as adenoma functional status and subtype.
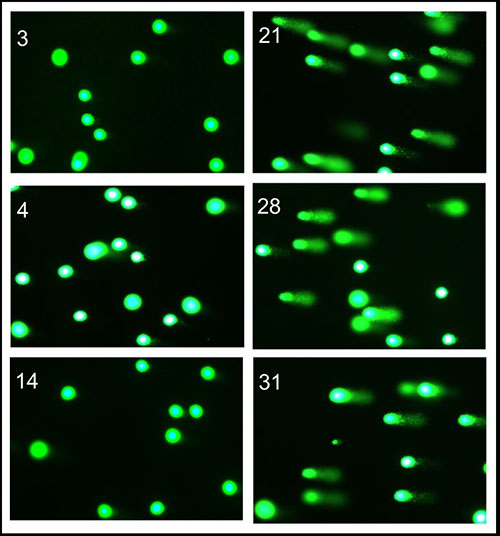
Figure 2. Comet assay and tail appearance (magnification ×20) in representative mice treated with PBS as control (left) or the long-acting GHRH analogue CJC-1295 (right).
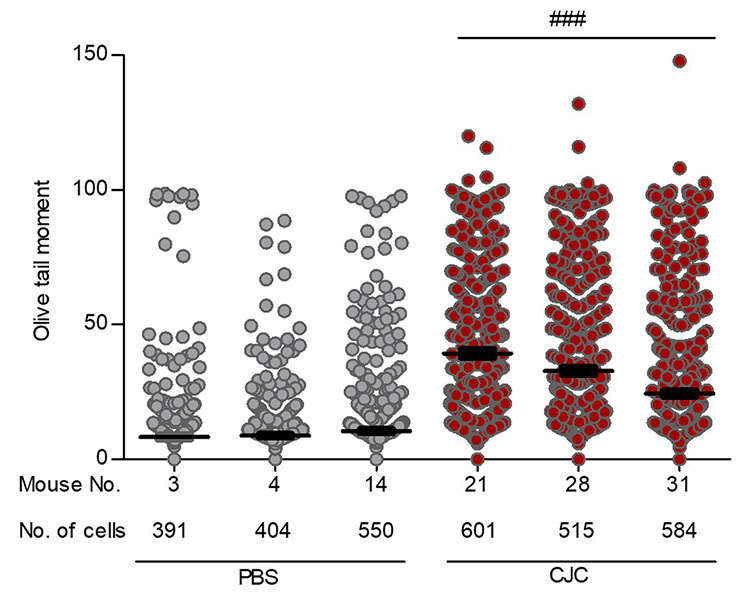
Figure 3. Comet assay Olive tail moment for the mice presented in Figure 2. The number of cells analyzed by a blinded observer is indicated. Results are presented as mean ± SEM. ###p≤0.001 for each CJC-1295–treated mouse versus each PBS-treated mouse by one-way ANOVA.
Novel Therapeutics for Aggressive Pituitary Adenomas
The Melmed Lab partners with the Pituitary Center to conduct laboratory, translational and clinical studies of novel therapeutics for aggressive pituitary adenomas. Following on studies of the epidermal growth factor receptor (EGFR) pathway in pituitary tumor growth, a phase II multicenter clinical trial demonstrated that the EGFR tyrosine kinase inhibitor lapatinib can reduce prolactin levels and tumor size in select patients with aggressive prolactinoma (Cooper et al., J Clin Endocrinol Metab. 2020). The CDK inhibitor seliciclib (R-roscovitine) was shown first in zebrafish, then in mouse and human pituitary cells, to suppress POMC gene expression and inhibit ACTH production, thereby reducing levels of circulating cortisol (Liu et al., J Clin Endocrinol Metab. 2015). An ongoing phase II multicenter clinical trial supported by the FDA Office of Orphan Products Development is evaluating the ability of seliciclib to normalize cortisol levels in patients with Cushing’s disease (Liu et al., J Clin Endocrinol Metab. 2023).
Novel Actions of Pituitary-Derived Exosomes
Cuiqi Zhou, PhD, conducts research on the novel actions of pituitary-derived exosomes, utilizing cellular and animal models as well as human iPSC-differentiated pituitary organoids. She elucidated pituitary exosome functions as non-hormonal messengers mediating intercellular communication, and identified exosome signatures derived from the pituitary as potential circulating biomarkers (Zhou et al., J Clin Endocrinol Metab. 2022).
Contact the Melmed Lab
8700 Beverly Blvd.
Davis Building, Room 3024
Los Angeles, CA 90048
