Research Areas
NLRP3 Inflammasome
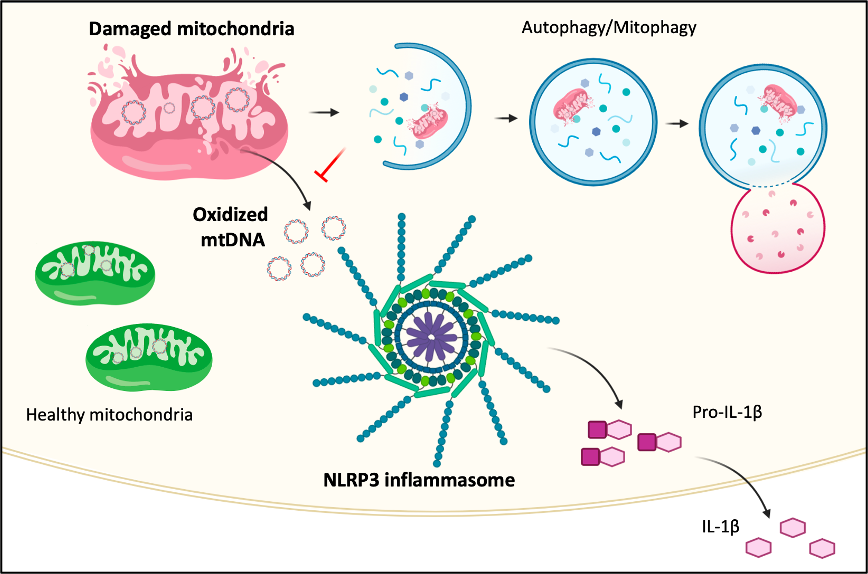
The NLRP3 inflammasome, a multi-complex that facilitates maturation of IL-1β (a critical proinflammatory cytokine involved in both acute and chronic inflammatory disease), is activated by many diverse danger signals. The Shimada Lab has found that the mitochondria are a central hub for the various NLRP3 activation signals. NLRP3 activators lead to mitochondrial dysfunction and apoptosis. During this process, mtROS is generated, which results in damaged (oxidized) mtDNA. The oxidized mtDNA is released into the cytosol where it binds to NLRP3 and activates the NLRP3 inflammasome. We are now studying the role of Ogg1, an oxidized DNA damage repair gene, in lung and vascular diseases.
Acute Lung Injury
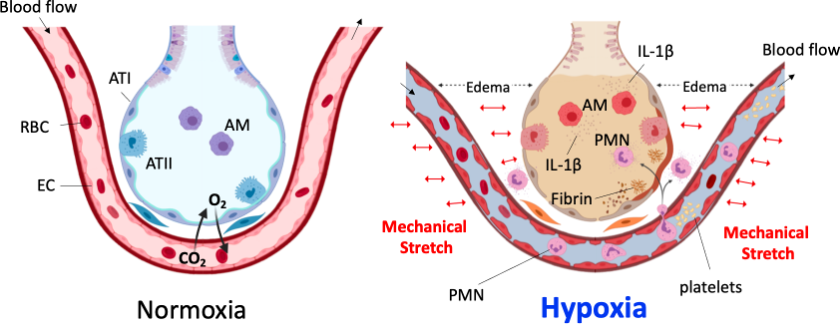
Acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome (ARDS), are syndromes of acute hypoxemic respiratory failure resulting from direct and indirect injuries to the gas exchange parenchyma of the lungs. Approximately 150,000-200,000 people are diagnosed with ARDS each year in the United States. In addition, 40% of severe and critical COVID-19 patients developed ARDS and, of those, over 50% died from the disease. LPS-induced ALI is an animal model that replicates key pathologic changes seen in ARDS, including loss of vascular integrity, neutrophil infiltration and accumulation of protein-rich fluid in the airspaces of the lung. Mechanical ventilation (MV) is one of the most frequently used interventions in the intensive care unit, and previous work has shown that the mechanical forces from this technique can enhance or perpetuate ventilator-induced ALI. We are now studying the role of IL-1 signaling in this context using a two-hit animal model that incorporates LPS-induced ALI with MV to gain insight into the molecular mechanisms of ARDS, and to identify potential therapeutic targets for its treatment. The Shimada Lab has recently reported that autophagy in myeloid cells protects IL-1β secretion in alveolar macrophages, thereby prevents developing two-hit inducing hypoxemia (Front. Immunol. 2020).
Contact the Shimada Lab
8700 Beverly Blvd.
Davis Building, Room 4008
Los Angeles, CA 90048