Breakthrough Research Areas
Expanding the Scope of Sex as a Biological Variable
Cancer disproportionately affects the male sex across racial, geographic and socioeconomic spectra; this is exemplified in bladder cancer in which more than 75% of patients are males. Many attributed this male bias to extrinsic factors such as environmental hazards and tobacco smoking, however, studies have shown that intrinsic biological factors such as sex hormones and sex chromosomes must also be playing a role.
Historically, the study of sex as a biological variable (SABV) overly emphasized gonadal sex. After decades of research, it is now more common to include the discussion of the chromosomal (genetic) sex in the study of SABV. At the Li Lab, we contributed to expanding this framework by verifying and defining the role of the chromosomal sex as well as other major sex-biasing factors, including epigenetic regulators and immune cells. Using a sex-reversed mouse model, we showed that sex chromosomes modulate bladder cancer risk independent of sex hormones. We also found that in bladder cancer, the hazard ratio of gonadal hormone effects (GHE) and sex chromosome effects (SCE) equaled the product rather than sum of its individual hazard ratio, suggesting synergy between the two. Overall, we expanded the study of SABV to include new sex-biasing agents; we also introduced the phenomenon that sex-biasing factors can have both independent and interactive functions. Only an accurate consideration of both independent and cross-talk effects between major sex-biasing factors can yield sustainable and effective clinical change for patients. To ensure this, we are proactively collaborating with labs at Cedars-Sinai and outside of Cedars-Sinai that specialize in expertise outside of our own to account for the whole picture of SABV.
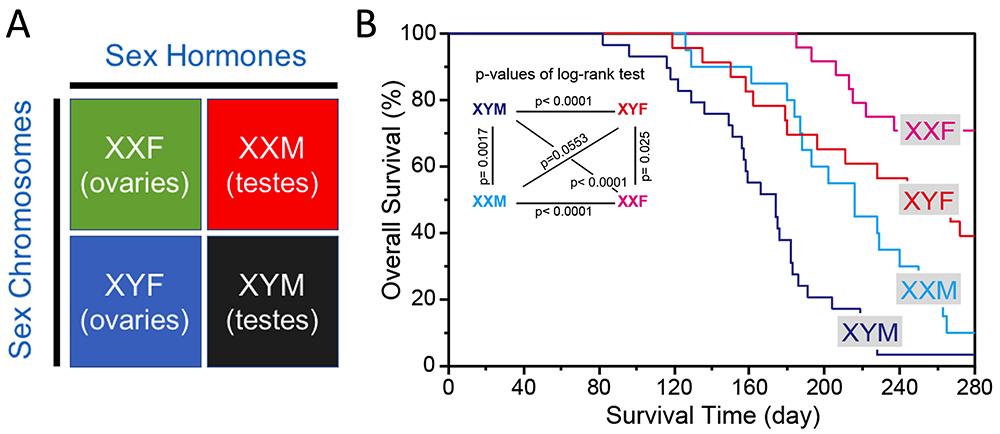
Both sex chromosomes (XX and XY) and gonads (ovaries and testes) exhibited independent and dependent sex-biasing effects. XX gonadal females (XXF) and XY gonadal males (XYM) showed the largest survival difference.
Discovery of Sex-Biased Epigenetics in Bladder Cancer
In 2018, we identified the first prototypical female-biased epigenetic regulator—lysine demethylase 6A (KDM6A)—suppressing bladder tumorigenesis. Our study found that urothelium-specific deletion of KDM6A reduced the baseline male-to-female bladder cancer risk ratio by more than fivefold, suggesting its function as a female-biased tumor suppressor. KDM6A conditional knockouts in males did not show worse survival, indicating that a paralog of KDM6A on the Y chromosome may be compensating loss of function KDM6A mutations on the X chromosome. KDM6A is an interesting gene for multiple reasons: (1) it is commonly mutated in bladder cancer, (2) it promotes expression of canonical Tp53 gene targets, (3) it is prone to escape X chromosome inactivation, resulting in an extra functional copy in females, and (4) it is a major epigenetic regulator. Based on our findings, we are pioneering the novel concept that the epigenetic sex or sex epigenome—the collective set of male and female sex-specific epigenetics—modulates bladder cancer risk and pathogenesis. Sex hormone and sex chromosome effects may alter the sex epigenome to render its sex-biasing effects. Characterizing the sex epigenome has many implications both in producing new lines of therapeutics for bladder cancer and in understanding the epigenetic basis of sex differences in diseases beyond bladder cancer.
Uncovering the Immunological Basis of Sex Differences
More recently, in collaboration with Ohio State’s Comprehensive Cancer Institute, we uncovered that androgen signaling promotes CD8+ T-cell exhaustion in males. This is a prime example of how sex hormones are not independently rendering sex-biasing effects. Cross-talk with immune cells has important implications on sex differences in immunity and immunotherapy. Existing clinical recommendations of immunotherapy for bladder cancer patients is not specific to biological sex. As stated in a recent press release, our goals are to: (1) further define the mechanism of how immunity is modulated in the male- and female-specific bladder tumor microenvironment and (2) use these findings to optimize timing and course of androgen-inhibitor therapy and immunotherapy for bladder cancer patients based on biological sex.
Stem Cells and Bladder Organogenesis
Our lab is also invested in defining bladder organogenesis and associated congenital anomalies. The urinary bladder is an evolutionary innovation unique to mammals, but its formation remains ill defined; human bladder agenesis or failure to form the bladder is a congenital birth defect that is also poorly understood. While most patients rarely survive, surviving patients exhibit defects in the iliac arteries, which are connected to the umbilical arteries during embryogenesis. Recently, our lab made headway in tying the developmental origin of the urinary bladder with the formation of umbilical arteries. We identified key molecular pathways that orchestrate the co-development of the bladder and umbilical arteries. Results provide novel implications on the evolution of the urinary bladder and potential causes for early pregnancy loss in humans relating to bladder agenesis.
The Lower Urinary Tract Development
We have put forward a new theory on how a single cloacal structure transforms into two separate entities: the lower urinary tract and the digestive tract. The new theory is based on the genetic lineage mapping data as well as comprehensive, high-resolution episcopic microscopic (HREM) 3D imaging data, and mouse disease models. This theory takes into account all structures involved in the cloaca morphogenesis and provides a new paradigm to understand the embryological basis of human anorectal and urogenital malformation. We also contributed to the understanding of the salient feature of bladder urothelium—quiescent, yet ready to regenerate upon injury. Using both chemical cyclophosphamide (CPP) and uropathogenic E. coli (UPEC) injury models, our recent findings suggest a new paradigm that epigenetic repression maintains bladder urothelium at the poised state to response rapidly to environmental cues.
Contact the X. Li Lab
8700 Beverly Blvd.
Davis Building, Suite 3090
Los Angeles, CA 90048