August 2025 Case
Clinical History
Patient in late 60’s who presented for evaluation of right breast calcifications detected on screening mammography. A subsequent core needle biopsy of the area showed atypical ductal hyperplasia (ADH), a radial scar, and lobular carcinoma in situ (LCIS). She subsequent underwent a right breast lumpectomy.
Histology
Histological examination revealed lobules containing cells arranged in a cribriform pattern with eosinophilic to basophilic, round to oval, collagen-rich spherules (in this case mucinous spherules - may be called mucinous spherperolosis instead). Adjacent regions demonstrated a monomorphic proliferation of mildly atypical cells with round nuclei, indistinct nucleoli, and dyshesive features, along with intracytoplasmic lumina. Other foci were composed of ducts with monomorphic cells displaying ovoid to rounded nuclei in a solid and cribriform pattern, each focus measuring less than 2 mm. A focal area displayed ducts filled with polymorphous cells with peripheral elongated clefts. Additional ducts exhibited an architecturally “flat” pattern without cytologic atypia. The epithelial lining cells were predominantly columnar, with elongated nuclei oriented perpendicular to the basement membrane.
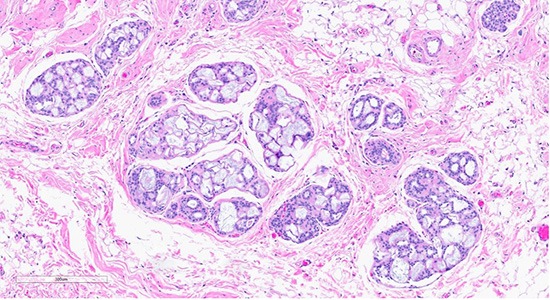
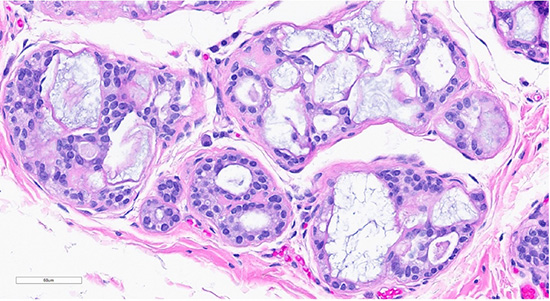
Figure 1: Microscopic slides of collagenous/mucinous spherulosis. Low-power (10× objective) H&E-stained section (top) shows cribriform architecture with collagenous/mucinous spherulosis. High-power (40× objective) H&E-stained section (bottom) demonstrates further detail, with myoepithelial cells surrounding round collagenous/mucinous spherules filled with intraluminal acellular basement membrane material.
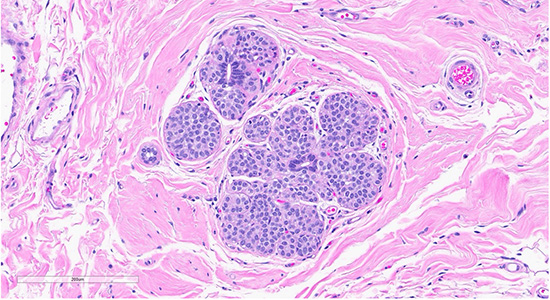
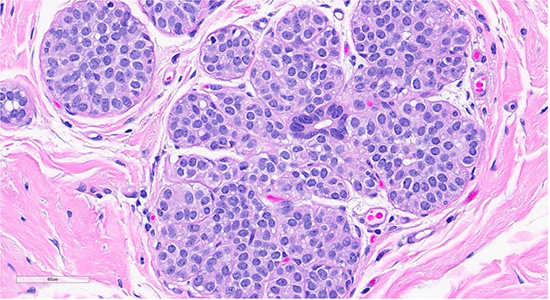
Figure 2: Scattered foci nearby the collagenous spherulosis exhibited mildly enlarged lobules filled with bland monomorphic cells (ALH/LCIS (top)., cellular dyshesion is better appreciated at high power (bottom).
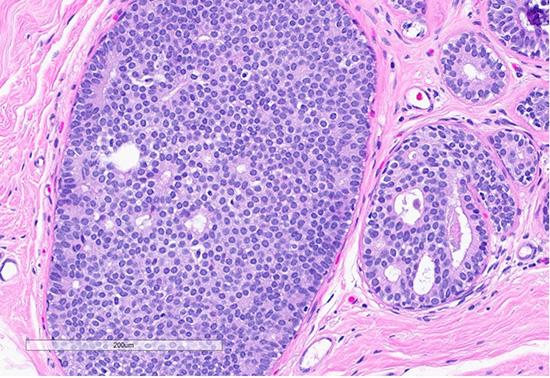
Figure 3: Microscopic slides of atypical ductal hyperplasia. The H&E section shows expanded ducts filled with monomorphic epithelial cells arranged in a mixed solid and cribriform pattern; the focus measures 2.5 mm
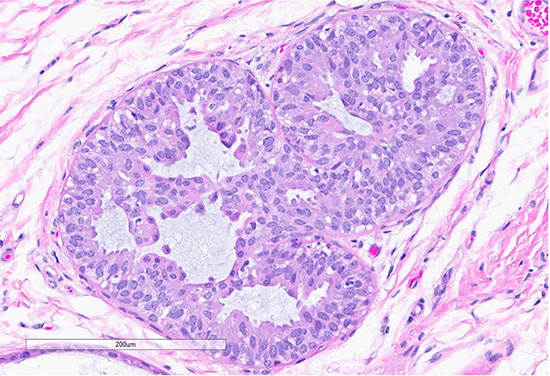
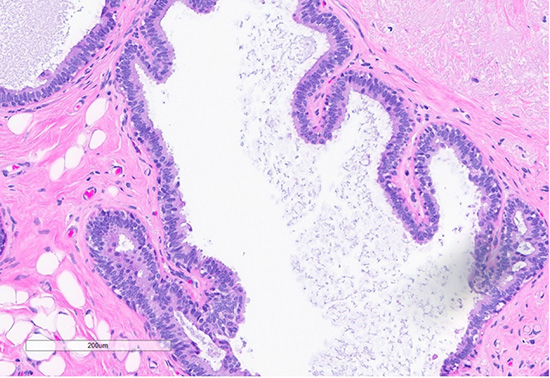
Figure 4: H&E section (top) shows a focus of usual ductal hyperplasia (UDH), characterized by expanded ducts filled with mildly polymorphous epithelial cells arranged in a haphazard pattern with irregular spaces. Scattered foci (bottom) exhibit features of columnar cell change, with the dilated ducts lined by columnar cells arranged perpendicularly to the basement membrane, with apical snouts.
Diagnosis
The excision specimen demonstrated multiple benign and atypical proliferative breast lesions, including atypical ductal hyperplasia, atypical lobular hyperplasia/lobular carcinoma in situ, usual ductal hyperplasia, columnar cell change, and collagenous spherulosis.
Discussion
Collagenous spherulosis (CS) is a rare, benign intraductal proliferative lesion of the breast1. It arises from the proliferation of basement membrane material, forming luminal hyaline spherules (eosinophilic to basophilic, occasionally mucoid, like this current case), surrounded by myoepithelial cells. These spherules contain a mixture of basement membrane components, banded collagen, and mineral deposits1.
Imaging features of collagenous spherulosis are variable and often incidental, as most cases are discovered alongside other benign breast lesions. On mammography, CS may appear as microcalcifications or, less commonly, as a mass, and architectural distortion is uncommon.
CS is commonly associated with other benign breast lesions such as sclerosing adenosis, papillomas, and radial scars. Notably, it can also be found in association with lobular carcinoma in situ (LCIS). Histologically, collagenous spherulosis can mimic malignant lesions such as adenoid cystic carcinoma (ACC), signet ring carcinoma, and cribriform pattern ductal carcinoma in situ (DCIS). ACC is a rare, low-grade basal-like carcinoma that shares histomorphologic features with CS. Molecular analysis can aid differentiation in which breast ACC frequently harbors recurrent MYB gene rearrangements, while CS and DCIS do not3,4.
Given that collagenous spherulosis is benign, no further clinical management is necessary if no other significant pathology is present, and patients may return to routine screening2. However, careful histologic evaluation is necessary as CS is often colonized by LCIS.
References
- Resetkova, E., Albarracin, C. & Sneige, N. Collagenous Spherulosis of Breast Morphologic Study of 59 Cases and Review of the Literature.
- Dimri, V. K. & Patel, A. K. Collagenous Spherulosis Presenting as Architectural Distortion on Mammography. J Breast Imaging 6, 332–333 (2024).
- Butcher, M. R., White, M. J., Rooper, L. M., Argani, P. & Cimino-Mathews, A. MYB RNA In Situ Hybridization Is a Useful Diagnostic Tool to Distinguish Breast Adenoid Cystic Carcinoma From Other Triple-Negative Breast Carcinomas. www.ajsp.com (2022).
- Warm, H. L. et al. Immunohistochemical marker profiles for the differentiation of collagenous spherulosis from adenoid cystic carcinoma of the breast. Hum Pathol 148, 7–13 (2024).