February 2021 Case
Authors
Wesley Rubenstein, DO (Fellow), Samuel Pepkowitz, MD (Faculty)
Transfusion Medicine
Clinical History
A male patient in his 50s with a history of hypertension presented with a chief complaint of chest pain and shortness of breath. CT scan at that time demonstrated a moderate to large pleural effusion with subtle nodularity. Video assisted thoracoscopic surgery (VATS) procedure was performed, and pathology work up of pleural and pericardial biopsies revealed metastatic adenocarcinoma of lung primary. PDL1 was noted to be <1% and an NGS panel was negative for alterations of EGFR, ALK, ROS, and MET. MRI brain without contrast showed evidence of brain/skull metastases.
The patient was treated with combination therapy consisting of pemetrexed, carboplatin, and pembrolizumab. The patient tolerated the therapy well and completed cycle #4 of combination therapy. A CT scan performed at that time demonstrated minimal change in the patient's hilar adenopathy and pleural thickening. He was given a 5th dose of pemetrexed and pembrolizumab followed by an additional dose of pemetrexed and carboplatin.
The next month he was admitted to an outside hospital with symptoms of altered mental status and was found to have a platelet count of 11,000/µL. Following further workup, he was diagnosed with TTP and treated with corticosteroids and daily therapeutic plasma exchange for 5 days before being discharged with a course of oral prednisone. The patient was also diagnosed with C. diff colitis a few days after initial presentation for which he was treated with antibiotics.
A few days after discharge, he presented to the ED with a complaint of fatigue, chills, and severe diarrhea. The patient stated that he had been compliant with his course of oral steroids while outpatient. His platelet count on admission was 135,000/µL but dropped to 21,000/µL on day 1 of admission. He was noted to have a coombs negative hemolytic anemia with hemoglobin 8.9 g/dL, LDH 477 U/L, haptoglobin 81 mg/dL, reticulocyte count 2.6%, and 1+ schistocytes on peripheral blood smear. Additional lab workup was remarkable for creatinine 1.41 mg/dL, serum calcium 8.2 mg/dL, D-dimer 1.6 µg/mL, and ADAMTS13 activity <5% with antibody level of 31 arbitrary units and inhibitor level of 0.7 inhibitor units. Infectious workup revealed a positive C. Diff toxin B, and a negative stool culture.
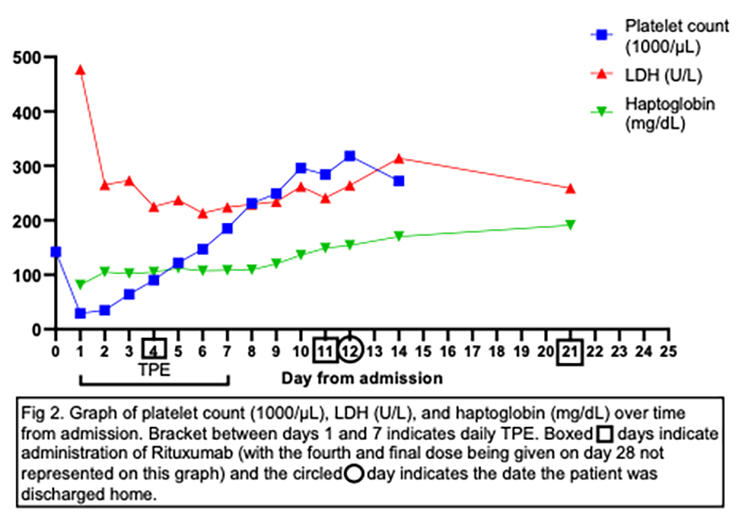
He was started on high dose dexamethasone and Transfusion Medicine was consulted for treatment of TTP with therapeutic plasma exchange. One plasma volume exchange using FFP was initiated on day 1 of admission and performed once daily until day 7 (7 consecutive sessions). Rituximab 375mg/m2 was initiated on day 4 and repeated on day 11. His platelet count (Fig. 2) and ADAMTS13 activity (Fig. 1) were monitored over the course of his treatment. He responded well to his treatment regimen and his platelets continued to trend up to a high of 318,000/µL on day 13 after admission when he was discharged home. He was continued on a 6-week outpatient dexamethasone taper with no relapse of symptoms and additional doses of Rituximab were given on days 9 and 16 from discharge for a total of 4 doses. Treatment of his lung cancer with pemetrexed monotherapy supplemented with vitamin B12 and folic acid was resumed 6 weeks following discharge and no relapse of TTP has occurred as of the completion of this report.

Fig. 1. ADAMTS13 activity, Antibody, and Inhibitor levels
Discussion
Programmed cell death 1 (PD-1) is a receptor expressed primarily on active T-cells and B-cells, that when bound to its associated ligand (PD-L1 or PD-L2), can counteract immune-stimulatory signals and prevent these cells from initiating an autoimmune response (1). Pembrolizumab is a humanized monoclonal IgG4 kappa isotype antibody against PD-1 that interferes with the PD-1/PD-L1 interaction and blocks inhibitory signals in T-cells which allows for recognition by cytotoxic T-cells (2). This interaction impairs a cancer cells ability to evade the immune system's antitumor response. Due to the immune upregulating effects of Pembrolizumab and other immune checkpoint inhibitors, various immune mediated side effects have been reported with these medications including colitis, dermatitis, hepatitis, pneumonitis, and immune mediated thrombocytopenia among others (3). This report will details a case of thrombotic thrombocytopenic purpura in a patient treated with Pembrolizumab.
Thrombotic thrombocytopenic purpura (TTP) is a potentially life-threatening condition caused by a deficiency of the ADAMTS13 expression or activity. ADAMTS13 is responsible for the cleavage of large prothrombotic VonWillebrand factor (vWF) multimers into smaller, less active forms. The deficiency of ADAMTS13, either inherited or acquired through an antibody mediated autoimmune response, results in the accumulation of large vWF multimers on endothelium that leads to platelet activation, adhesion, and ultimately consumptive depletion. Diffuse uncontrolled platelet activation and adhesion can lead to microangiopathic hemolytic anemia and severe activation can cause small vessel occlusion resulting in end organ damage.
The impetus for acquired TTP is often unknown, but can be the result of infection, malignancy, medications, or preexisting autoimmune disorders. Given the immune stimulatory effects of Pembrolizumab and its propensity to cause autoimmune conditions, it is reasonable to consider that Pembrolizumab contributed to the onset of TTP in this patient. In 2019, Dickey et al. reported a case of TTP, confirmed by ADAMTS13 activity <3%, developing in a patient following her 5th dose of Pembrolizumab for treatment of metastatic non-small cell lung cancer. The patient’s Pembrolizumab therapy was stopped, and she was treated with 5 days of plasma exchange and IV methylprednisolone with eventual normalization of her platelet count and resolution of microangiopathic hemolytic anemia. Several additional studies have implicated the use of other immune checkpoint inhibitors such as nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor) in the development of TTP, associated with a reduced ADAMTS13 activity and improvement following therapeutic plasma exchange and cessation of checkpoint inhibitor therapy [4-7].
The patient in this report, in contrast to the other reported case involving pembrolizumab, had fewer comorbidities, a higher ADAMTS13 activity level at cessation of TPE (77% vs 35%), and had rituximab included in his therapy regimen, which all may have contributed to a longer period of post treatment remission. A drop in ADAMTS13 activity level post-treatment for TTP has been linked to increased risk of TTP relapse [13]. Current treatment guidelines suggest monitoring platelet levels as an indication of treatment efficacy, but it may be possible to achieve improved remission rates in high-risk patients if treatment is continued until both platelet and ADAMTS13 levels are returned to baseline prior to discontinuation of plasma exchange.
Therapeutic plasma exchange is already recommended as a category I, grade 1A treatment for TTP by the American Society for Apheresis [14], but it is likely especially important in the treatment of TTP induced by Pembrolizumab, which has a half-life of 26 days [15] and could continue to exert its effect following initial TTP treatment. Adjunct immunosuppressive therapy such as corticosteroids and rituximab are also commonly used to help maintain disease remission as was the case for the patient in this report.
Another treatment option for TTP approved by the FDA in 2019 for the treatment of acquired TTP is the monoclonal antibody Caplacizumab which blocks the interaction between vWF and platelet glycoprotein-Ib thereby preventing the formation of pathologic microthrombi. Clinical trials have demonstrated reduced morbidity and mortality in patients treated with Caplacizumab compared to those treated with only standard therapy of daily plasma exchange until at least 2 days after platelet count normalization, steroids, and other immunosuppressive therapy. The difference in mortality is hypothesized by the study authors to be due to the prevention of end organ ischemic damage while immunosuppressive therapy takes effect [16]. One consequence of the use of this treatment and its blockade of platelet consumption is that platelet count can no longer be used as a surrogate of disease response to therapy so other measures such as ADAMTS13 activity, inhibitor, or antibody levels could provide more valuable insight into treatment efficacy and deciding when to stop TPE in these cases.
In addition to Pembrolizumab, the patient was treated with carboplatin and pemetrexed which are approved for first line treatment of lung adenocarcinoma. Pemetrexed has been suspected as a possible cause of TTP in prior case reports [11] by a mechanism that requires further investigation. It is also known that cancer itself is associated with thrombotic microangiopathic anemia (TMA) but this is usually seen at the onset or recurrence of the malignancy and the patient in this report appeared to be in remission when his diagnosis of TTP was made [12].
The patient in this report was prescribed amlodipine, which has been reported in some cases as a cause of autoimmune thrombocytopenia [8], for treatment of hypertension; however, an associated between TTP and amlodipine treatment has not been reported. The other medications in the patient's regimen are not known to be associated with TTP or other autoimmune conditions. The patient was diagnosed with C. diff colitis and treated with vancomycin during his first hospitalization at an outside hospital which have both been suspected to have a possible association with TTP and thrombotic microangiopathic anemia [9, 10], but these are not thought to be likely causes of TTP in this patient since his infection and subsequent treatment with vancomycin did not occur until 4 days following the initial finding of severe thrombocytopenia and admission to the hospital.
In conclusion, we report a case of TTP following treatment with the PD-1 inhibitor Pembrolizumab. To date, there is a scarcity of TTP cases related to immune checkpoint inhibitor therapy reported in the literature with this case being only the second one involving Pembrolizumab and the first to demonstrate complete remission following treatment. Further studies will be needed to determine the prognostic implications of immune checkpoint inhibitor associated TTP and whether standard TTP therapy is optimal for treating this entity as well.
References
- Zhao, P., Wang, P., Dong, S. et al. Depletion of PD-1-positive cells ameliorates autoimmune disease. Nat Biomed Eng 3, 292–305 (2019). https://doi.org/10.1038/s41551-019-0360-0
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn M-J, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015; 372:2018–2028.
- Dickey MS, Raina AJ, Gilbar PJ, Wisniowski BL, Collins JT, Karki B, Nguyen AD. Pembrolizumab-induced thrombotic thrombocytopenic purpura. J Oncol Pharm Pract. 2020 Jul;26(5):1237-1240. doi: 10.1177/1078155219887212. Epub 2019 Nov 13. PMID: 31718453.
- Ali Z, Zafar MU, Wolfe Z, Akbar F, Lash B. Thrombotic Thrombocytopenic Purpura Induced by Immune Checkpoint Inhibitiors: A Case Report and Review of the Literature. Cureus. 2020 Oct 29;12(10):e11246. doi: 10.7759/cureus.11246. PMID: 33274128; PMCID: PMC7707147.
- King J, de la Cruz J, Lutzky J. Ipilimumab-induced thrombotic thrombocytopenic purpura (TTP). J Immunother Cancer. 2017 Mar 21;5:19. doi: 10.1186/s40425-017-0224-7. PMID: 28344807; PMCID: PMC5360069.
- Lancelot, M, Miller, MJ, Roback, J, Stowell, SR. Refractory thrombotic thrombocytopenic purpura related to checkpoint inhibitor immunotherapy. Transfusion. 2020; 1– 7. https://doi.org/10.1111/trf.16117
- Youssef A, Kasso N, Torloni AS, Stanek M, Dragovich T, Gimbel M, Mahmoud F. Thrombotic Thrombocytopenic Purpura due to Checkpoint Inhibitors. Case Rep Hematol. 2018 Dec 20;2018:2464619. doi: 10.1155/2018/2464619. PMID: 30671268; PMCID: PMC6317083.
- Garbe E, Meyer O, Andersohn F, Aslan T, Kiesewetter H, Salama A. Amlodipine-induced immune thrombocytopenia. Vox Sang. 2004 Jan;86(1):75-6. doi: 10.1111/j.0042-9007.2004.00376.x. PMID: 14984564.
- Terrero Salcedo, DA; Guevara-Hernandez, MA; Terrero Salcedo, MA. THROMBOTIC THROMBOCYTOPENIC PURPURA AFTER AN EPISODE OF SEVERE CLOSTRIDIUM DIFFICILE COLITIS. Abstract published at Hospital Medicine 2017, May 1-4, 2017; Las Vegas, Nev. Abstract 751. Journal of Hospital Medicine Volume 12 Suppl 2.
- Reese JA, Bougie DW, Curtis BR, Terrell DR, Vesely SK, Aster RH, George JN. Drug-induced thrombotic microangiopathy: Experience of the Oklahoma Registry and the BloodCenter of Wisconsin. Am J Hematol. 2015 May;90(5):406-10. doi: 10.1002/ajh.23960. Epub 2015 Feb 25. PMID: 25639727; PMCID: PMC4409501.
- Alabiso, I., Baratelli, C., Brizzi, M.P. et al. Acute and fatal thrombocytopenic thrombotic purpura after a single dose of pemetrexed. Int J Clin Pharm 36, 1141–1143 (2014). https://doi.org/10.1007/s11096-014-0032-9
- Oberic L, Buffet M, Schwarzinger M, Veyradier A, Clabault K, Malot S, Schleinitz N, Valla D, Galicier L, Bengrine-Lefèvre L, Gorin NC, Coppo P; Reference Center for the Management of Thrombotic Microangiopathies. Cancer awareness in atypical thrombotic microangiopathies. Oncologist. 2009 Aug;14(8):769-79. doi: 10.1634/theoncologist.2009-0067. Epub 2009 Aug 14. PMID: 19684072.
- von Auer-Wegener C, Schmidtmann I, Bach L, et al. Longitudinal assessment of ADAMTS13-activity helps predict recurrence of immune thrombotic thrombocytopenic purpura (iTTP): results from the German TTP-registry. Abstract OC 07.4. Presented at ISTH 2020 Virtual Congress; July 12-14, 2020.
- Padmanabhan A, ConnellySmith L, Aqui N, et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice – Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apher. 2019;34: 171–354. https://doi.org/10.1002/jca.21705
- Dang TO, Ogunniyi A, Barbee MS, Drilon A. Pembrolizumab for the treatment of PD-L1 positive advanced or metastatic non-small cell lung cancer. Expert Rev Anticancer Ther. 2016;16(1):13-20. doi:10.1586/14737140.2016.1123626
- Hanlon A, Metjian A. Caplacizumab in adult patients with acquired thrombotic thrombocytopenic purpura. Ther Adv Hematol. 2020;11:2040620720902904. Published 2020 Feb 7. doi:10.1177/2040620720902904