July 2025 Case
Authors
Matthew Leong, MD (Fellow), Mahzad Azimpouran (Resident), Benjamin Barrena (Fellow), Matthew Gayhart, MD (Faculty)
Molecular Pathology
Clinical History
Patient in his late 80’s with a history of type 2 diabetes, hypertension, hypothyroidism, and recurrent small bowel obstructions, presented for evaluation of an ill-defined 3.0cm mass of his left foot which he first noticed 6 months ago. There is mild tenderness over the mass, rated 4/10. An initial ultrasound-guided biopsy was performed and a month later the mass was excised.
Histology
Histological examination demonstrated an infiltrative cellular proliferation of spindle and pleomorphic cells, with mild to moderate cytologic atypia and no necrosis (Fig 1). Mitotic figures were not seen.
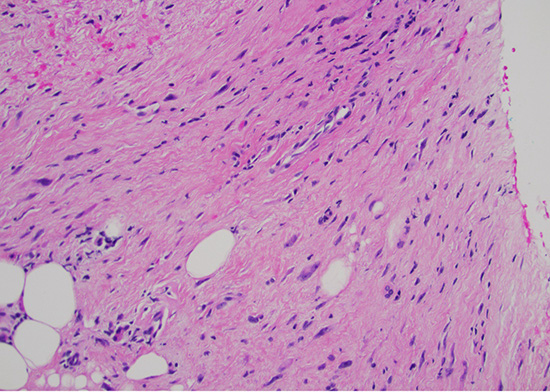
Figure 1: H&E stain at 200x magnification displaying mild to moderate cytologic atypia without necrosis or mitoses.
Ancillary Studies
Immunohistochemistry (IHC) was performed with results summarized in Table 1. Given the nonspecific morphological and immunophenotype, additional molecular testing was performed, including MDM2 fluorescent in-situ hybridization (FISH), in-house next-generation sequencing (NGS), and send-out to Cleveland Clinic for solid tumor gene fusion NGS.
Antibody |
Result |
|---|---|
|
Smooth muscle actin (SMA) |
Partially positive |
|
SOX10 |
Negative |
|
Desmin |
Negative |
|
MDM2 |
Negative |
|
CD34 |
Partially positive |
|
Retinoblastoma |
Partially lost |
|
INI1/SMARCB1 |
Retained |
|
Epithelial membrane antigen (EMA) |
Negative |
|
Pancytokeratin (AE1/AE3) |
Negative |
|
BCL-1 (Cyclin D1) |
Partially positive |
Table 1: Summary of IHC results
Diagnosis
Atypical spindle and pleomorphic cell proliferation, best interpreted as a low-grade sarcoma. On the excision specimen, the tumor measured 3.3 cm in greatest dimension and involved the dermis, adipose tissue, fascia, and was present at the surgical margins.
Molecular Findings
In-house NGS did not detect any clinically relevant molecular alterations and FISH did not identify any MDM2 gene amplification; however, the send-out fusion NGS detected a novel YAP1::FUS fusion protein. The fusion occurred between YAP1 exon 4 and FUS exon 2.
Discussion
YAP1 is a crucial transcriptional regulator which provides downstream transcriptional regulation in the Hippo signaling pathway, which physiologically functions to regulate organ size and tissue repair.1 Within this pathway, YAP1 promotes cell proliferation while suppressing apoptotic genes through interactions with a series of transcription factors, most notably through TEAD (transcriptional enhanced associate domain) transcription factors. As such, it is implicated in tumorigenesis, contributing to initiation, progression, and therapy resistance in numerous cancer types.2
While the exact pathogenic role of YAP1 is not fully understood, it does not appear able to independently drive malignant transformation, as simple overexpression of the protein did not lead to tumor formation. Additional inactivation of upstream Hippo tumor suppressors or multiple activating point mutations are usually required to initiate transformation.3–5 Missense mutations have been reported in the serine residues which control regulation, with the most important being S127 and S397 which regulates nuclear exclusion and proteasomal degradation, respectively.
YAP1 fusions, on the other hand, have evidence showing they can independently function as tumor initiating events and oncogenic drivers.5,7–10 These fusions lead to the production of a chimeric protein with the N-terminal domain of the YAP1 protein fused to the C-terminal domain of another protein. These fusion proteins preserve the TEAD-binding domain of the YAP1 protein but will lose the regulatory S397 residue, impairing degradation and leading to accumulation of YAP1 protein. Most of the YAP1 fusion partners possess putative transactivation or p300 interaction domains which are theorized to act as surrogate for lost YAP1 C-terminal transactivation domains.5 Reported fusion partners include MAMLD1, FAM118B, TFE3, and SS18. Of note, the YAP1::FUS fusion in this case has not yet been previously reported.
Due to the emerging role of YAP1 as an oncogenic driver, it is currently being evaluated as a potential biomarker for targeted therapy. Some of the most promising studies are looking into small molecule inhibitors that impede the YAP1-TEAD interactions, as this is a shared function in all studied fusion proteins thus far.12–15
References
- Wang, Y., Yu, A. & Yu, F.-X. The Hippo pathway in tissue homeostasis and regeneration. Protein Cell 8, 349–359 (2017).
- Zhang, W. et al. YAP promotes malignant progression of Lkb1-deficient lung adenocarcinoma through downstream regulation of survivin. Cancer Res 75, 4450–7 (2015).
- Zhao, B., Li, L., Tumaneng, K., Wang, C.-Y. & Guan, K.-L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev 24, 72–85 (2010).
- Zhao, B., Li, L., Lei, Q. & Guan, K.-L. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev 24, 862–74 (2010).
- Szulzewsky, F. et al. Comparison of tumor-associated YAP1 fusions identifies a recurrent set of functions critical for oncogenesis. Genes Dev 34, 1051–1064 (2020).
- Zhang, X. et al. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat Commun 8, 15280 (2017).
- Rosenbaum, E. et al. Prognostic stratification of clinical and molecular epithelioid hemangioendothelioma subsets. Mod Pathol 33, 591–602 (2020).
- Pajtler, K. W. et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 27, 728–43 (2015).
- Pajtler, K. W. et al. YAP1 subgroup supratentorial ependymoma requires TEAD and nuclear factor I-mediated transcriptional programmes for tumorigenesis. Nat Commun 10, 3914 (2019).
- Takadera, M. et al. Phenotypic characterization with somatic genome editing and gene transfer reveals the diverse oncogenicity of ependymoma fusion genes. Acta Neuropathol Commun 8, 203 (2020).
- Szulzewsky, F., Holland, E. C. & Vasioukhin, V. YAP1 and its fusion proteins in cancer initiation, progression and therapeutic resistance. Dev Biol 475, 205–221 (2021).
- Brodowska, K. et al. The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation. Exp Eye Res 124, 67–73 (2014).
- Pobbati, A. V et al. Targeting the Central Pocket in Human Transcription Factor TEAD as a Potential Cancer Therapeutic Strategy. Structure 23, 2076–86 (2015).
- Song, S. et al. A Novel YAP1 Inhibitor Targets CSC-Enriched Radiation-Resistant Cells and Exerts Strong Antitumor Activity in Esophageal Adenocarcinoma. Mol Cancer Ther 17, 443–454 (2018).
- Bum-Erdene, K. et al. Small-Molecule Covalent Modification of Conserved Cysteine Leads to Allosteric Inhibition of the TEAD⋅Yap Protein-Protein Interaction. Cell Chem Biol 26, 378-389.e13 (2019).