June 2021 Case
Authors
Manuel J. Arana Rosainz, MD, PhD (Fellow), Eric Vail, MD (Faculty)
Molecular Genetic Pathology
Clinical History
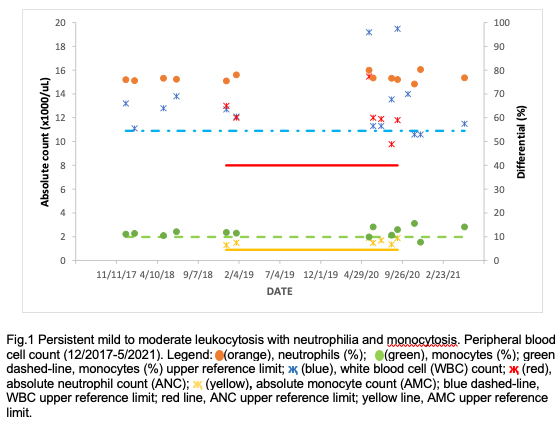
This patient is a male in his 50’s with persistent leukocytosis with neutrophilia and monocytosis. Patient reported sustained leukocytosis for many years. For the past 8 months, a mild increase in white blood cell (WBC) counts has been noted (13.6x109/L [11.1-19.18x109/L]), as well as a moderate increase in absolute neutrophil count (ANC) (12.9 x109/L [9.8-15.4 x109/L]) and absolute monocyte count (AMC) (1.6 x109/L [1.36-1.9x109/L]) with lymphocyte, eosinophil, and basophil counts within normal limits (Fig. 1). Peripheral blood smear examination revealed neutrophilia with reactive changes. Molecular studies were ordered for assessment of myeloproliferative neoplasm (MPN) due to persistent mild WBC count alterations, although no other clinical or laboratory findings suggestive of MPN or hematopoietic neoplasms were found.
Molecular Analysis
Molecular profiling of the patient's peripheral blood was performed using the Cedars-Sinai comprehensive myeloid panel (CMP) that uses Archer VariantPlex, which is a targeted amplicon-based NGS assay that includes 75 genes frequently mutated in myeloid malignancies. DNA sequencing revealed a frameshift truncating alteration in CXCR4, Y11Ifs*5 (VAF = 0.51). No other clinically relevant mutations were detected. Also, quantitative Reverse Transcriptase PCR for BCR-ABL1 fusion did not detected a fusion transcript.
Discussion
This patient molecular profile detected an inactivating CXCR4 variant. Inactivating CXCR4 (C-X-C chemokine receptor, type 4) mutations have not been associated with myeloid neoplasia. Nevertheless, several lines of evidence suggest that CXCR4 haploinsufficiency could cause mobilization of all leukocyte subsets from bone marrow and reduced neutrophil homing from blood to bone marrow, possibly resulting in persistent leukocytosis, as observed in this patient.
CXCR4 is a G protein–coupled receptor for the chemokine CXCL12 that promotes bone marrow homing and retention of neutrophils and hematopoietic stem cells (HSCs) (1). CXCR4 gain-of-function mutations result from the truncation of the C-terminus, a region responsible for negative regulation of the receptor. CXCR4 C-terminus mutants disrupt the negative regulatory elements in the carboxy-terminus, exaggerating the normal hematopoietic functions of the receptor, including HSC and neutrophil retention in the bone marrow, neutrophil homing to bone marrow from blood, and HSC differentiation into committed myeloid progenitors (2,3).
Approximately 30%–40% of patients with Waldenstrom’s macroglobulinemia (WM) carry heterozygous somatic mutations of CXCR4 (4). In addition, heterozygous pathogenic CXCR4 germline variants are responsible for WHIM syndrome (OMIM #193670; warts, hypogammaglobulinemia, infections, and myelokathexis), a combined immunodeficiency disease, which includes panleukopenia and severe neutropenia in most cases (5,6). Somatic mutations in WM resemble WHIIM syndrome activating germline variants, which disrupt the negative regulatory elements in the receptor C-terminus, as mentioned above. Interestingly, a case report described a patient cured of WHIM syndrome by chromothripsis that fortuitously deleted the diseased CXCR4 allele, resulting in overcorrection of leukopenia in the neutrophil and monocyte lineages (7).
Although CXCR4 Y11Ifs*5 is neither reported nor functionally characterized in the literature, it may result in loss of function of CXCR4 protein. This inactivating CXCR4 alteration was observed in this patient at an allele frequency consistent with a germline mutation, though the Cedars-Sinai CMP is not validated to differentiate between germline and somatic mutations. Based on the patient's history and the NGS results, leukocytosis associated with CXCR4 haploinsufficiency is suggested in this case. In mouse models, WBC count was increased by CXCR4 haploinsufficiency, which reduced neutrophil homing from blood to bone marrow (8). Furthermore, it has been demonstrated that the inhibition of CXCR4 signaling with a specific antagonist, mimicking CXCR4 haploinsufficiency, caused mobilization of all leukocyte subsets from bone marrow to blood and prevented the homing of aged neutrophils back to bone marrow for clearance (9,10).
In this patient, a likely germline inactivating CXCR4 mutation could explain the presence of persistent mild to moderate leukocytosis with moderate neutrophilia and monocytosis. Follow-up germline testing is recommended for confirmation. Additional testing to rule out other low suspicion disorders, less commonly associated with mild persistent leukocytosis, are also suggested if clinically warranted.
References
- Lear T, Dunn SR, McKelvey AC, Mir A, Evankovich J, Chen BB, Liu Y. RING finger protein 113A regulates C-X-C chemokine receptor type 4 stability and signaling. Am J Physiol Cell Physiol. 2017 Nov 1;313(5):C584-C592. doi: 10.1152/ajpcell.00193.2017. Epub 2017 Oct 4. Erratum in: Am J Physiol Cell Physiol. 2019 Apr 1;316(4):C582. PMID: 28978524; PMCID: PMC5792167.
- Venkatesan S, Rose JJ, Lodge R, Murphy PM, Foley JF. Distinct mechanisms of agonist-induced endocytosis for human chemokine receptors CCR5 and CXCR4. Mol Biol Cell. 2003 Aug;14(8):3305-24. doi: 10.1091/mbc.e02-11-0714. Epub 2003 May 3. PMID: 12925765; PMCID: PMC181569.
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006 Dec;25(6):977-88. doi: 10.1016/j.immuni.2006.10.016. PMID: 17174120.
- Scala S, D'Alterio C, Milanesi S, Castagna A, Carriero R, Farina FM, Locati M, Borroni EM. New Insights on the Emerging Genomic Landscape of CXCR4 in Cancer: A Lesson from WHIM. Vaccines (Basel). 2020 Apr 3;8(2):164. doi: 10.3390/vaccines8020164. PMID: 32260318; PMCID: PMC7349554.
- Gulino AV. WHIM syndrome: a genetic disorder of leukocyte trafficking. Curr Opin Allergy Clin Immunol. 2003 Dec;3(6):443-50. doi: 10.1097/00130832-200312000-00005. PMID: 14612668.
- Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003 May;34(1):70-4. doi: 10.1038/ng1149. PMID: 12692554..
- McDermott DH, Gao JL, Liu Q, Siwicki M, Martens C, Jacobs P, Velez D, Yim E, Bryke CR, Hsu N, Dai Z, Marquesen MM, Stregevsky E, Kwatemaa N, Theobald N, Long Priel DA, Pittaluga S, Raffeld MA, Calvo KR, Maric I, Desmond R, Holmes KL, Kuhns DB, Balabanian K, Bachelerie F, Porcella SF, Malech HL, Murphy PM. Chromothriptic cure of WHIM syndrome. Cell. 2015 Feb 12;160(4):686-699. doi: 10.1016/j.cell.2015.01.014. Epub 2015 Feb 5. PMID: 25662009; PMCID: PMC4329071.
- Gao JL, Yim E, Siwicki M, Yang A, Liu Q, Azani A, Owusu-Ansah A, McDermott DH, Murphy PM. Cxcr4-haploinsufficient bone marrow transplantation corrects leukopenia in an unconditioned WHIM syndrome model. J Clin Invest. 2018 Aug 1;128(8):3312-3318. doi: 10.1172/JCI120375. Epub 2018 Jun 25. PMID: 29715199; PMCID: PMC6063486.
- McDermott DH, Liu Q, Velez D, Lopez L, Anaya-O'Brien S, Ulrick J, Kwatemaa N, Starling J, Fleisher TA, Priel DA, Merideth MA, Giuntoli RL, Evbuomwan MO, Littel P, Marquesen MM, Hilligoss D, DeCastro R, Grimes GJ, Hwang ST, Pittaluga S, Calvo KR, Stratton P, Cowen EW, Kuhns DB, Malech HL, Murphy PM. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014 Apr 10;123(15):2308-16. doi: 10.1182/blood-2013-09-527226. Epub 2014 Feb 12. PMID: 24523241; PMCID: PMC3983611.
- Devi S, Wang Y, Chew WK, Lima R, A-González N, Mattar CN, Chong SZ, Schlitzer A, Bakocevic N, Chew S, Keeble JL, Goh CC, Li JL, Evrard M, Malleret B, Larbi A, Renia L, Haniffa M, Tan SM, Chan JK, Balabanian K, Nagasawa T, Bachelerie F, Hidalgo A, Ginhoux F, Kubes P, Ng LG. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med. 2013 Oct 21;210(11):2321-36. doi: 10.1084/jem.20130056. Epub 2013 Sep 30. PMID: 24081949; PMCID: PMC3804935.