Research Areas
The Bottini Lab studies mechanisms of systemic autoimmunity. We are particularly interested in projects arising from observations made on patient blood and tissues, which could lead to the identification of novel drug targets or markers for personalized disease prediction and/or treatment.
Understanding Protein Phosphatases Encoded by Autoimmunity Genes
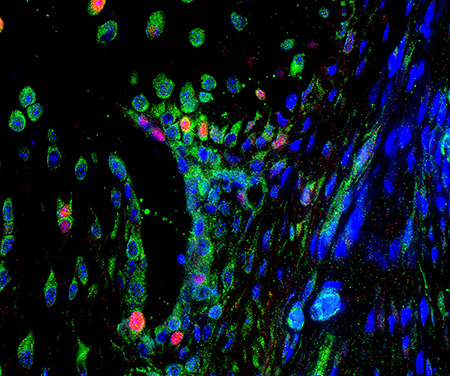
Fluorescent collagen deposition assay applied to bleomycin model of skin fibrosis.
Why Do We Work in This Area?
Autoimmune diseases are caused by a complex combination of genetic and environmental factors. While genetic studies have identified many autoimmunity genes, the mechanisms of action of most of these genes in human autoimmunity is currently unknown. Understanding the effects of disease-associated genetic variations on gene function is important because it could potentially pave the way for targeting these genes or other components within their pathways for innovative therapies. Such therapies could be tailored to individual patients, addressing the specific mechanisms underlying disease manifestation in individuals carrying these gene variants.
What Do We Do in This Area?
The laboratory investigates the in vivo immunological mechanisms, as well as the biochemical regulation and cellular functions, of two protein phosphatases: PTPN22 and PTPN2, encoded by significant autoimmunity genes. Dr. Bottini reported the initial discovery that identified PTPN22 as a major autoimmunity risk gene. Currently, PTPN22 ranks as the second most important gene in rheumatoid arthritis, and it also increases the risk of systemic lupus erythematosus, systemic sclerosis, inflammatory bowel disease (IBD), and type 1 diabetes.
Targeting Protein Phosphatases for Fibrosis
Why Do We Work in This Area?
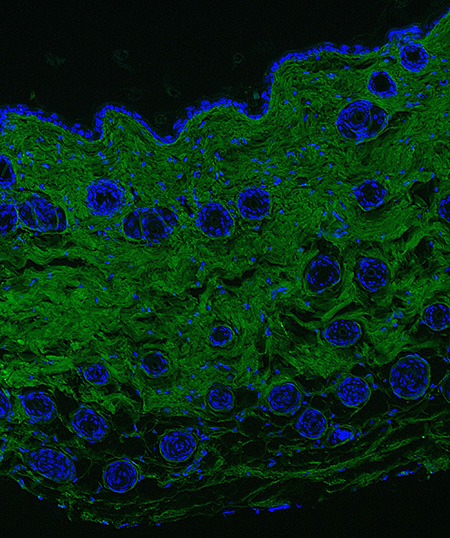
Fluorescent image of arthritic joint synovial lining.
Scleroderma is a severe autoimmune disease that leads to scarring of the skin and internal organs (referred to as “fibrosis"). Unfortunately, most patients are diagnosed at a stage of disease when some fibrosis of the skin and the internal organs has already occurred. In systemic lupus erythematosus, scarring of the skin and kidney fibrosis (called “nephrosclerosis”) are severe complications of cutaneous lupus and lupus nephritis. Rheumatoid arthritis can be complicated by severe scarring of the lungs. There is a critical need for medications that can significantly reduce progression of and reverse fibrosis in scleroderma, rheumatoid arthritis and lupus. Fibrosis results from multiple cellular and molecular abnormalities in the tissue microenvironment (see below), but a key downstream effector of fibrosis is the fibroblast. Targeting phosphatase signaling in fibroblasts or other cells could modify the disease course in scleroderma and potentially other fibrotic diseases such as idiopathic pulmonary fibrosis and liver fibrosis.
What Do We Do in This Area?
Protein phosphatases are powerful regulators of most biochemical signaling pathways inside cells, yet their role in fibroblasts has remained largely unexplored. Through published and preliminary experiments, we found that fibroblasts express several protein phosphatases. We are now focused on studying these molecules and assessing whether and how they can be targeted for novel anti-fibrotic therapies.
Rheumatoid Arthritis and Scleroderma Tissue Microenvironment Studies
Why Do We Work in This Area?
In autoimmune diseases, there has been emphasis on known immunological mechanisms to determine their critical importance in tissue inflammation. If these mechanisms are deemed significant, efforts are made to discover or apply agents capable of suppressing them (known as immunosuppressants). This approach has worked relatively well in rheumatoid arthritis, for which we have several immunosuppressive disease-modifying medications which are effective although they increase the risk of infections and potentially cancer. However, the immunosuppressant-based approach has been much less successful in scleroderma and other autoimmune diseases. Also, a signficiant number of rheumatoid arthritis patients do not respond to or stop responding to immunosuppressants.
Recently, there has been an increased focus on the microenvironment of affected tissues in autoimmunity (“the issue is in the tissue”). Together with the development of sophisticated single cell and spatial genomic approaches, these studies are showing that there are tissue and patient-specific alterations in cell phenotypes, cell-cell cross talk and spatial issues within affected tissues. A deeper knowledge of these complex and tissue-specific alterations and how they drive disease will lead to therapies that address the local problem. Such treatments will be safer and more personalized as they correct specific tissue alterations in selected patients without affecting the immunological mechanisms that defend us from infections and cancer.
What Do We Do in This Area?
We study local inflammation in the joints and lungs of rheumatoid arthritis patients and in the skin and lungs of scleroderma patients. We make hypotheses and test them in cell biology systems and animal models of disease. In rheumatoid arthritis, we work on interactions between local fibroblast-like cells, called synoviocytes, and immune cells. Although synoviocytes play a disease-promoting role, they are not immune cells. Therefore inhibiting synoviocyte functions would not result in the general suppression of the immune system. We study biochemical signaling pathways inside synoviocytes and how synoviocytes modulate the phenotype of T cells in the joints to render them more pathogenic.
In scleroderma, we are carrying out spatial biology studies on skin and lung tissues generously donated by our patients. We also collaborate with other KAI investigators to understand whether alterations found in studies of patient blood samples, followed longitudinally in the Kao Multispecialty Scleroderma Program, correlate with and drive pathologic changes in scleroderma tissues. Finally, we collaborate strictly with an immunoengineering laboratory to explore novel technologies to locally restore the health of the immune system in rheumatoid arthritis and scleroderma and treat disease without causing generalized immunosuppression.
Contact the Bottini Lab
121 North San Vicente Boulevard, Room 211
Beverly Hills, CA 90211
